当前位置:
X-MOL 学术
›
Food Funct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of linear epitopes and their major role in the immunoglobulin E-binding capacity of tropomyosin from Alectryonella plicatula
Food & Function ( IF 5.1 ) Pub Date : 2022-08-09 , DOI: 10.1039/d2fo01713j Nai-Ru Ji 1 , Xin-Yu Han 1 , Chen-Chen Yu 1 , Xin-Rong He 1 , Shi-Tao Rao 2 , Fei Huan 1 , Hong Liu 1 , Gui-Xia Chen 3 , Min-Jie Cao 1 , Guang-Ming Liu 1
Food & Function ( IF 5.1 ) Pub Date : 2022-08-09 , DOI: 10.1039/d2fo01713j Nai-Ru Ji 1 , Xin-Yu Han 1 , Chen-Chen Yu 1 , Xin-Rong He 1 , Shi-Tao Rao 2 , Fei Huan 1 , Hong Liu 1 , Gui-Xia Chen 3 , Min-Jie Cao 1 , Guang-Ming Liu 1
Affiliation
|
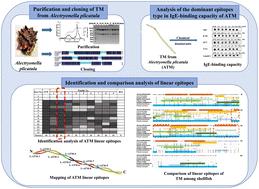
Tropomyosin (TM) is an important allergen in molluscans. However, there was a lack of information about TM as an allergen in oysters. TM was purified and identified from Alectryonella plicatula (ATM), and its primary sequence was cloned and encoded with 284 amino acids (AAs). Chemical denaturants were used to destroy the structure to confirm that linear epitopes played a major role in the immunoglobulin E-binding capacity of ATM. Subsequently, nine linear epitopes were identified using a serological test. The peptide with AA27–41 was regarded as the key epitope because it could be recognized strongly by most sera of oyster-sensitive individuals in comparison to other epitope peptides. Finally, the epitopes and the primary sequence of TM among shellfish were aligned to find the two conserved epitopes (AA117–132 and AA164–178) in oyster, octopus, abalone, scallop, clam, shrimp, and crab. Overall, these data provide a foundation for the allergenicity and cross-reactivity of TM.
中文翻译:

线性表位的鉴定及其在 Alectryonella plicatula 原肌球蛋白免疫球蛋白 E 结合能力中的主要作用
原肌球蛋白 (TM) 是软体动物中一种重要的过敏原。然而,缺乏关于 TM 作为牡蛎过敏原的信息。TM是从Alectryonella plicatula (ATM)中纯化鉴定的,其一级序列被克隆并编码284个氨基酸(AAs)。化学变性剂用于破坏结构,以确认线性表位在 ATM 的免疫球蛋白 E 结合能力中起主要作用。随后,使用血清学测试鉴定了九个线性表位。AA 27–41的肽被认为是关键表位,因为与其他表位肽相比,它可以被大多数牡蛎敏感个体的血清强烈识别。最后,通过比对贝类中TM的表位和一级序列,找到牡蛎、章鱼、鲍鱼、扇贝、蛤、虾和蟹中的两个保守表位(AA 117-132和AA 164-178)。总体而言,这些数据为 TM 的过敏性和交叉反应性提供了基础。
更新日期:2022-08-09
中文翻译:

线性表位的鉴定及其在 Alectryonella plicatula 原肌球蛋白免疫球蛋白 E 结合能力中的主要作用
原肌球蛋白 (TM) 是软体动物中一种重要的过敏原。然而,缺乏关于 TM 作为牡蛎过敏原的信息。TM是从Alectryonella plicatula (ATM)中纯化鉴定的,其一级序列被克隆并编码284个氨基酸(AAs)。化学变性剂用于破坏结构,以确认线性表位在 ATM 的免疫球蛋白 E 结合能力中起主要作用。随后,使用血清学测试鉴定了九个线性表位。AA 27–41的肽被认为是关键表位,因为与其他表位肽相比,它可以被大多数牡蛎敏感个体的血清强烈识别。最后,通过比对贝类中TM的表位和一级序列,找到牡蛎、章鱼、鲍鱼、扇贝、蛤、虾和蟹中的两个保守表位(AA 117-132和AA 164-178)。总体而言,这些数据为 TM 的过敏性和交叉反应性提供了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号