Minerals Engineering ( IF 4.9 ) Pub Date : 2022-08-07 , DOI: 10.1016/j.mineng.2022.107770 Evgeniy Kuzas , Denis Rogozhnikov , Oleg Dizer , Kirill Karimov , Andrei Shoppert , Alexey Suntsov , Ivan Zhidkov
|
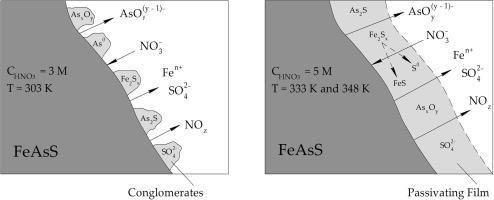
The kinetics of arsenopyrite compact samples dissolution in nitric acid media was investigated using the full factorial experiment model (FFEM). The effects of nitric acid concentration (2–5 M), temperature (303–348 K), disk rotation frequency (3.33–10 rps) on the arsenopyrite dissolution rate were studied. Nitric acid concentration and temperature have a significant effect, and disk rotation frequency does not. With an increase in nitric acid concentration from 4 M to 5 M and temperature from 318 K to 333 K, the dissolution rates of iron and arsenic increase by 30.1–30.4 times, from 0.26–0.27 × 10–6 mol dm−2 s−1 to 7.83–8.21 × 10–6 mol dm−2 s−1. Increasing temperature to 348 K does not lead to a significant increase in the dissolution rate. The data obtained were processed using the FFEM to obtain kinetic equations (R2 = 0.95): , . Using SEM–EDS and XPS analyses, the mechanism of arsenopyrite passivation was determined. Under mild conditions (nitric acid concentration of 3 M and temperature of 303 K), local conglomerates containing iron polysulphides (Fe2Sx) are formed on the arsenopyrite surface. They do not create diffusion difficulties when dissolving arsenopyrite. Under harsh conditions (nitric acid concentration of 5 M and temperature of 333 K), polysulphides begin to aggregate. It leads to the formation of the passivating film containing elemental sulphur. The film is thin (20–30 μm) and porous. While the arsenopyrite dissolution rate is high. Further increase of parameters leads to thickening and compaction of the passivating film and decrease in the arsenopyrite dissolution rate.
中文翻译:

转盘法毒砂在硝酸介质中溶解动力学研究
使用全因子实验模型 (FFEM) 研究了毒砂致密样品在硝酸介质中溶解的动力学。研究了硝酸浓度(2-5 M)、温度(303-348 K)、圆盘旋转频率(3.33-10 rps)对毒砂溶解速率的影响。硝酸浓度和温度有显着影响,而圆盘旋转频率则没有。随着硝酸浓度从 4 M 增加到 5 M,温度从 318 K 增加到 333 K,铁和砷的溶解速率增加了 30.1-30.4 倍,从 0.26-0.27 × 10 -6 mol dm -2 s - 1至 7.83–8.21 × 10 –6 mol dm -2 s -1. 将温度升高到 348 K 不会导致溶解速率显着增加。使用 FFEM 处理获得的数据以获得动力学方程 (R 2 = 0.95):,. 使用 SEM-EDS 和 XPS 分析,确定了毒砂钝化的机制。在温和条件下(3 M 的硝酸浓度和 303 K 的温度),毒砂表面会形成含有多硫化铁 (Fe 2 S x ) 的局部砾岩。它们在溶解毒砂时不会造成扩散困难。在苛刻的条件下(5 M 的硝酸浓度和 333 K 的温度),多硫化物开始聚集。它导致形成含有元素硫的钝化膜。该薄膜很薄(20-30 μm)且多孔。而毒砂溶解率高。参数的进一步增加导致钝化膜的增厚和压实以及毒砂溶解速率的降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号