Journal of Controlled Release ( IF 10.5 ) Pub Date : 2022-08-08 , DOI: 10.1016/j.jconrel.2022.07.025 Moritz Radbruch 1 , Hannah Pischon 1 , Fang Du 2 , Rainer Haag 2 , Fabian Schumacher 3 , Burkhard Kleuser 3 , Lars Mundhenk 1 , Achim D Gruber 1
|
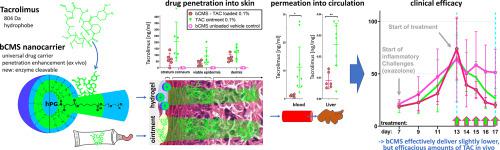
Two challenges in topical drug delivery to the skin include solubilizing hydrophobic drugs in water-based formulations and increasing drug penetration into the skin. Polymeric core-multishell nanocarrier (CMS), particularly the novel biodegradable CMS (bCMS = hPG-PCL1.1K-mPEG2k-CMS) have shown both advantages on excised skin ex vivo. Here, we investigated topical delivery of tacrolimus (TAC; > 500 g/mol) by bCMS in a hydrogel on an oxazolone-induced model of dermatitis in vivo. As expected, bCMS successfully delivered TAC into the skin. However, in vivo they did not increase, but decrease TAC penetration through the stratum corneum compared to ointment. Differences in the resulting mean concentrations were mostly non-significant in the skin (epidermis: 35.7 ± 20.9 ng/cm2 for bCMS vs. 92.6 ± 62.7 ng/cm2 for ointment; dermis: 76.8 ± 26.8 ng/cm2 vs 118.2 ± 50.4 ng/cm2), but highly significant in blood (plasma: 1.1 ± 0.4 ng/ml vs 11.3 ± 9.3 ng/ml; erythrocytes: 0.5 ± 0.2 ng/ml vs 3.4 ± 2.4 ng/ml) and liver (0.01 ± 0.01 ng/mg vs 0.03 ± 0.01 ng/mg). bCMS were detected in the stratum corneum but not in viable skin or beyond. The therapeutic efficacy of TAC delivered by bCMS was equivalent to that of standard TAC ointment. Our results suggest that bCMS may be a promising carrier for the topical delivery of TAC. The quantitative difference to previous results should be interpreted in light of structural differences between murine and human skin, but highlights the need as well as potential methods to develop more a complex ex vivo analysis on human skin to ensure quantitative predictive value.
中文翻译:

可生物降解核-多壳纳米载体:他克莫司局部给药治疗皮炎
将局部药物输送到皮肤的两个挑战包括在水基制剂中溶解疏水性药物和增加药物对皮肤的渗透。聚合物核-多壳纳米载体 (CMS),特别是新型可生物降解 CMS (bCMS = hPG-PCL 1.1K -mPEG 2k -CMS) 在离体切除的皮肤上显示出两种优势。在这里,我们在恶唑酮诱导的体内皮炎模型上研究了通过 bCMS 在水凝胶中局部递送他克莫司(TAC;> 500 g/mol)。正如预期的那样,bCMS 成功地将 TAC 输送到皮肤中。然而,在体内与软膏相比,它们没有增加,但减少了 TAC 通过角质层的渗透。所得平均浓度的差异在皮肤中大多不显着(表皮:bCMS为 35.7 ± 20.9 ng/cm 2与软膏为92.6 ± 62.7 ng/cm 2;真皮:76.8 ± 26.8 ng/cm 2 与118.2 ± 50.4 ng/cm 2),但在血液(血浆:1.1 ± 0.4 ng/ml vs 11.3 ± 9.3 ng/ml;红细胞:0.5 ± 0.2 ng/ml vs 3.4 ± 2.4 ng/ml)和肝脏(0.01 ± 0.01 ng/mg对比0.03 ± 0.01 纳克/毫克)。在角质层中检测到 bCMS,但在有活力的皮肤或更远的地方没有检测到。bCMS 提供的 TAC 的治疗效果与标准 TAC 软膏相当。我们的结果表明,bCMS 可能是 TAC 局部给药的有前途的载体。应根据小鼠和人类皮肤之间的结构差异来解释与先前结果的定量差异,但强调需要以及潜在的方法来开发对人类皮肤进行更复杂的离体分析以确保定量预测值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号