Water Research ( IF 11.4 ) Pub Date : 2022-08-05 , DOI: 10.1016/j.watres.2022.118953 Yumeng Qi 1 , Nannan Wu 1 , Zhengnan Tu 1 , Virender K Sharma 2 , Zhongbo Wei 1 , Dongmei Zhou 1 , Zunyao Wang 1 , Ruijuan Qu 1
|
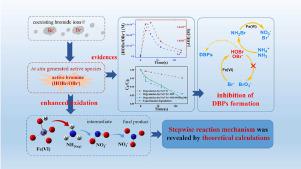
This work systematically examined the capability of ferrate (Fe(VI)) for ammonia oxidation, revealing for the first time that bromide ions (Br−) played an important role in promoting the removal of ammonia in Fe(VI) system. In the presence of 10.0 mM Br−, the removal efficiency of ammonia was nearly 3.4 times that of the control, and 1.0 mM ammonia was almost completely removed after two rounds addition of 1.0 mM Fe(VI) in 60 min. PMSO probe test, electron paramagnetic resonance spectra and radical quenching experiments were employed to interpret the underlying promotion mechanism of Br−, and it was proposed that the formation of active bromine (HOBr/OBr−) played a dominant role in the enhanced oxidative removal of ammonia by Fe(VI). Further kinetic model simulations revealed that HOBr/OBr− and Fe(VI) were the two major reactive species in Fe(VI)/Br− system, accounting for 66.7% and 33.0% of ammonia removal, respectively. As the target contaminant, ammonia could quickly consume the generated HOBr/OBr−, thereby suppressing the formation of brominated disinfection byproducts. Finally, NO3− was identified as the dominant transformation product of ammonia, and density functional theory (DFT) calculations revealed that six reaction stages were involved in ammonia oxidation with the first step as the rate-limiting step. This work would enable the full use of coexisting bromides for effective removal of ammonia from natural waters or wastewaters by in situ Fe(VI) oxidation method.
中文翻译:

Fe(VI)/Br−氧化系统中氨的去除增强:动力学、转化机制和理论计算
本工作系统地考察了高铁酸盐(Fe(VI))对氨的氧化能力,首次揭示了溴离子(Br - )在促进Fe(VI)体系中氨的去除中发挥了重要作用。在 10.0 mM Br -存在下,氨的去除效率几乎是对照的 3.4 倍,在 60 分钟内加入两轮 1.0 mM Fe(VI) 后,1.0 mM 氨几乎完全去除。采用PMSO探针测试、电子顺磁共振光谱和自由基猝灭实验来解释Br -的潜在促进机制,提出活性溴(HOBr/OBr -) 在 Fe(VI) 对氨的增强氧化去除中起主要作用。进一步的动力学模型模拟表明,HOBr/OBr -和 Fe(VI) 是 Fe(VI)/Br -体系中的两种主要活性物质,分别占氨去除的 66.7% 和 33.0%。作为目标污染物,氨可以快速消耗生成的 HOBr/OBr -,从而抑制溴化消毒副产物的形成。最后,NO 3 -被确定为氨的主要转化产物,密度泛函理论(DFT)计算表明氨氧化涉及六个反应阶段,第一步是限速步骤。这项工作将能够充分利用共存的溴化物通过原位Fe(VI) 氧化法从天然水或废水中有效去除氨。











































 京公网安备 11010802027423号
京公网安备 11010802027423号