Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2022-08-08 , DOI: 10.1016/j.apcatb.2022.121827 Jéssica A. de Oliveira , Jean C. da Cruz , Otaciro R. Nascimento , Caue Ribeiro
|
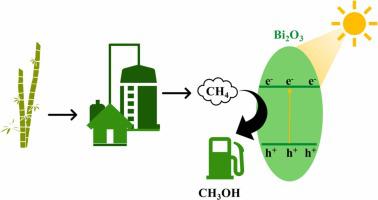
Herein, we propose that an efficient CH4 partial photooxidation to methanol depends on appropriate band edge positions in photocatalysts. We demonstrated our hypothesis using Bi2O3 since this semiconductor have a valence band favorable to produce •OH and a conduction band not advantageous to O2•- which is crucial to keep O2 available for •CH3 capture and CH3OH formation. A notably and selective partial photooxidation of methane to methanol was observed under visible light at room temperature and pressure. As a result, the productivity of methanol over Bi2O3 can reach approximately 3771 μmol g−1 h−1 with c.a. 65% selectivity avoiding overoxidation to CO2. Isotope labeling experiment (13CH4) confirmed that methane acts as the carbon source of methanol and ESR measurements proved the •CH3 and •OH generation. Besides, Bi2O3 exhibited good stability after 5 cycles maintaining a high and selective methanol production. These results provide insight into critical photocatalyst properties for the partial photooxidation of methane to methanol.
中文翻译:

在室温和压力下通过 Bi2O3 部分氧化选择性 CH4 重整为甲醇
在此,我们提出有效的 CH 4部分光氧化成甲醇取决于光催化剂中适当的带边缘位置。我们使用Bi 2 O 3证明了我们的假设,因为这种半导体具有有利于产生•OH 的价带和不利于O 2 •的导带——这对于保持O 2可用于•CH 3捕获和CH 3 OH 形成至关重要. 在室温和压力下,在可见光下观察到甲烷显着且选择性地部分光氧化为甲醇。结果,甲醇的产率超过 Bi 2 O 3可以达到大约3771 μmol g -1 h -1,选择性约为65%,避免了过氧化成CO 2。同位素标记实验( 13 CH 4 )证实甲烷是甲醇的碳源,ESR测量证明了•CH 3和•OH的生成。此外,Bi 2 O 3在5个循环后表现出良好的稳定性,保持了高选择性的甲醇产量。这些结果提供了对甲烷部分光氧化成甲醇的关键光催化剂性能的深入了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号