当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(III)-Catalyzed Tandem Reaction Access to (Quinazolin-2-yl)methanone Derivatives from 2,1-Benzisoxazoles and α-Azido Ketones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-08 , DOI: 10.1021/acs.joc.2c01214 Shan Liu 1 , An-Jing Wang 1 , Min Li 1 , Jing Zhang 1 , Guo-Dong Yin 2 , Wen-Ming Shu 1, 2 , Wei-Chu Yu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-08 , DOI: 10.1021/acs.joc.2c01214 Shan Liu 1 , An-Jing Wang 1 , Min Li 1 , Jing Zhang 1 , Guo-Dong Yin 2 , Wen-Ming Shu 1, 2 , Wei-Chu Yu 1
Affiliation
|
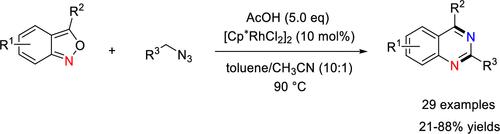
A Rh(III)-catalyzed tandem reaction for the synthesis of (quinazolin-2-yl)methanone derivatives has been explored from 2,1-benzisoxazoles and α-azido ketones. The transformation involves Rh(III)-catalyzed denitrogenation of α-azido ketones, aza-[4 + 2] cycloaddition, ring opening, and dehydration aromatization processes. Notably, the aza-[4 + 2] cycloaddition of an imine rhodium complex intermediate with 2,1-benzisoxazoles is the key to this reaction.
中文翻译:

Rh(III)-催化串联反应从 2,1-苯并异恶唑和 α-叠氮基酮获得 (Quinazolin-2-yl)methanone 衍生物
已经探索了从 2,1-苯并异恶唑和 α-叠氮基酮合成 (quinazolin-2-yl)methanone 衍生物的 Rh(III) 催化的串联反应。该转化涉及 Rh(III) 催化的 α-叠氮基酮脱氮、氮杂-[4 + 2] 环加成、开环和脱水芳构化过程。值得注意的是,亚胺铑络合物中间体与 2,1-苯并异恶唑的氮杂-[4 + 2] 环加成反应是该反应的关键。
更新日期:2022-08-08
中文翻译:

Rh(III)-催化串联反应从 2,1-苯并异恶唑和 α-叠氮基酮获得 (Quinazolin-2-yl)methanone 衍生物
已经探索了从 2,1-苯并异恶唑和 α-叠氮基酮合成 (quinazolin-2-yl)methanone 衍生物的 Rh(III) 催化的串联反应。该转化涉及 Rh(III) 催化的 α-叠氮基酮脱氮、氮杂-[4 + 2] 环加成、开环和脱水芳构化过程。值得注意的是,亚胺铑络合物中间体与 2,1-苯并异恶唑的氮杂-[4 + 2] 环加成反应是该反应的关键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号