当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Correlation of Pazopanib in (Ethanol/n-Propanol/2-Propanol/1-Butanol + Acetonitrile) Mixtures from T = 288.15 to 328.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-08-08 , DOI: 10.1021/acs.jced.1c00548 Yongjun Wu 1, 2 , Hongwei Shi 1, 3, 4 , Yong Xie 1, 3, 4 , Jun Zhu 1, 3, 4 , Cong Wang 1, 3, 4 , Hongyan Wang 1, 3, 4
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-08-08 , DOI: 10.1021/acs.jced.1c00548 Yongjun Wu 1, 2 , Hongwei Shi 1, 3, 4 , Yong Xie 1, 3, 4 , Jun Zhu 1, 3, 4 , Cong Wang 1, 3, 4 , Hongyan Wang 1, 3, 4
Affiliation
|
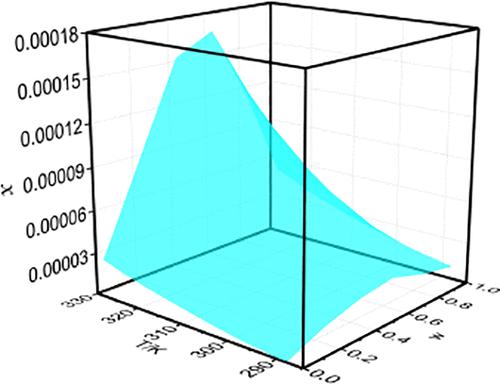
The solubility data of pazopanib in four mixed solvents including acetonitrile (1) + ethanol (2), acetonitrile (1) + n-propanol (2), acetonitrile (1) + 2-propanol (2), and acetonitrile (1) + 1-butanol (2) binary mixtures at temperatures ranging from 288.15 to 328.15 K was obtained using the isothermal saturation method under 101.3 kPa. The quantification of pazopanib was determined by using high-performance liquid-phase chromatograph (HPLC). In pure solvents, the largest solubility was obtained in 1-butanol followed by n-propanol, isopropanol, ethanol, and acetonitrile. The solubility in alcohols increased with the increase in carbon chain length. In addition, pazopanib is easier to combine with alcohol than acetonitrile. However, the solubility data all increased first and then decreased with the increasing mass fraction of alcohols in four mixed solvents. In the mixture of ethanol (w) + acetonitrile (1 – w), the maximum and minimum values at 328.15 K were 2.576 × 10–4 and 2.722 × 10–5, respectively, and observed at w = 0.60 and pure acetonitrile, with the increase of 9.46 folds. However, in the system of isopropanol (w) + acetonitrile (1 – w), the largest data was obtained at w = 0.40, 1.212 × 10–4 at 328.15 K. In the two other mixtures, the largest solubility were all found at w = 0.60 with the mass fraction of alcohols. The solubility data in four binary mixtures was correlated by three models. In addition, the residual sum of squares (RSS) is 4.81 × 10–9 and the square of linear correlation coefficient is 0.996, which is very close to 1. The Jouyban–Acree model could be used to correlate the solubility values of pazopanib in the selected four mixed solvents. More importantly, the experimental data and model parameters are useful in the preparation and purification of pazopanib.
中文翻译:

帕唑帕尼在 T = 288.15 至 328.15 K 的(乙醇/正丙醇/2-丙醇/1-丁醇 + 乙腈)混合物中的溶解度测量和相关性
帕唑帕尼在乙腈(1)+乙醇(2)、乙腈(1)+正丙醇(2)、乙腈(1)+2-丙醇(2)、乙腈(1)+四种混合溶剂中的溶解度数据使用等温饱和法在 101.3 kPa 下获得温度范围为 288.15 至 328.15 K 的 1-丁醇 (2) 二元混合物。帕唑帕尼的定量采用高效液相色谱仪(HPLC)测定。在纯溶剂中,在 1-丁醇中的溶解度最大,其次是n-丙醇、异丙醇、乙醇和乙腈。在醇中的溶解度随着碳链长度的增加而增加。此外,帕唑帕尼比乙腈更容易与酒精结合。然而,溶解度数据均随着醇在四种混合溶剂中质量分数的增加而先增加后减少。在乙醇 (w) + 乙腈 (1 – w ) 的混合物中,在 328.15 K 处的最大值和最小值分别为 2.576 × 10 –4和 2.722 × 10 –5,在w = 0.60 和纯乙腈时观察到,增加了 9.46 倍。然而,在异丙醇(w)+乙腈(1- w)的体系中,最大的数据是在w= 0.40,在 328.15 K 时为 1.212 × 10 –4。在另外两种混合物中,最大溶解度均在w = 0.60 时发现,醇的质量分数。四种二元混合物中的溶解度数据通过三个模型相关联。此外,残差平方和 (RSS) 为 4.81 × 10 –9,线性相关系数的平方为 0.996,非常接近 1。Jouyban-Acree 模型可用于关联帕唑帕尼在选用四种混合溶剂。更重要的是,实验数据和模型参数可用于帕唑帕尼的制备和纯化。
更新日期:2022-08-08
中文翻译:

帕唑帕尼在 T = 288.15 至 328.15 K 的(乙醇/正丙醇/2-丙醇/1-丁醇 + 乙腈)混合物中的溶解度测量和相关性
帕唑帕尼在乙腈(1)+乙醇(2)、乙腈(1)+正丙醇(2)、乙腈(1)+2-丙醇(2)、乙腈(1)+四种混合溶剂中的溶解度数据使用等温饱和法在 101.3 kPa 下获得温度范围为 288.15 至 328.15 K 的 1-丁醇 (2) 二元混合物。帕唑帕尼的定量采用高效液相色谱仪(HPLC)测定。在纯溶剂中,在 1-丁醇中的溶解度最大,其次是n-丙醇、异丙醇、乙醇和乙腈。在醇中的溶解度随着碳链长度的增加而增加。此外,帕唑帕尼比乙腈更容易与酒精结合。然而,溶解度数据均随着醇在四种混合溶剂中质量分数的增加而先增加后减少。在乙醇 (w) + 乙腈 (1 – w ) 的混合物中,在 328.15 K 处的最大值和最小值分别为 2.576 × 10 –4和 2.722 × 10 –5,在w = 0.60 和纯乙腈时观察到,增加了 9.46 倍。然而,在异丙醇(w)+乙腈(1- w)的体系中,最大的数据是在w= 0.40,在 328.15 K 时为 1.212 × 10 –4。在另外两种混合物中,最大溶解度均在w = 0.60 时发现,醇的质量分数。四种二元混合物中的溶解度数据通过三个模型相关联。此外,残差平方和 (RSS) 为 4.81 × 10 –9,线性相关系数的平方为 0.996,非常接近 1。Jouyban-Acree 模型可用于关联帕唑帕尼在选用四种混合溶剂。更重要的是,实验数据和模型参数可用于帕唑帕尼的制备和纯化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号