当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed native α-amino acid derivative-directed arylation/oxidation of benzylic C–H bonds: synthesis of 5-aryl-1,4-benzodiazepin-2-ones
Chemical Communications ( IF 4.3 ) Pub Date : 2022-08-08 , DOI: 10.1039/d2cc03266j Fengxuan Jiang 1, 2 , Menghua Xu 3 , Wenfeng Bei 1 , Kai Cheng 1 , Lehao Huang 2
Chemical Communications ( IF 4.3 ) Pub Date : 2022-08-08 , DOI: 10.1039/d2cc03266j Fengxuan Jiang 1, 2 , Menghua Xu 3 , Wenfeng Bei 1 , Kai Cheng 1 , Lehao Huang 2
Affiliation
|
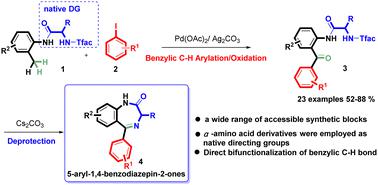
A Pd-catalyzed, native α-amino acid derivative-directed benzylic C–H bond arylation/oxidation with aryl iodides was developed. The natural amino acid auxiliary could serve as a desired building block for formation of 5-aryl-1,4-benzodiazepin-2-ones after removal of the trifluoroacetyl protecting group. The bifunctional reaction probably proceeded through a sequential benzylic arylation/oxidation process.
中文翻译:

钯催化的天然 α-氨基酸衍生物定向芳基化/苄基 C-H 键的氧化:5-aryl-1,4-benzodiazepin-2-ones 的合成
开发了一种 Pd 催化的、天然 α-氨基酸衍生物导向的苄基 C-H 键芳基化/芳基碘化物氧化。在去除三氟乙酰基保护基团后,天然氨基酸助剂可用作形成 5-aryl-1,4-benzodiazepin-2-ones 的所需结构单元。双功能反应可能通过连续的苄基芳基化/氧化过程进行。
更新日期:2022-08-08
中文翻译:

钯催化的天然 α-氨基酸衍生物定向芳基化/苄基 C-H 键的氧化:5-aryl-1,4-benzodiazepin-2-ones 的合成
开发了一种 Pd 催化的、天然 α-氨基酸衍生物导向的苄基 C-H 键芳基化/芳基碘化物氧化。在去除三氟乙酰基保护基团后,天然氨基酸助剂可用作形成 5-aryl-1,4-benzodiazepin-2-ones 的所需结构单元。双功能反应可能通过连续的苄基芳基化/氧化过程进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号