European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2022-08-07 , DOI: 10.1016/j.ejmech.2022.114633 Nazariy Pokhodylo 1 , Nataliya Finiuk 2 , Olha Klyuchivska 3 , Mykola A Тupychak 1 , Vasyl Matiychuk 1 , Evgeny Goreshnik 4 , Rostyslav Stoika 2
|
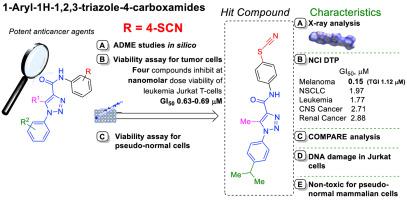
The N-(4-thiocyanatophenyl)-1H-1,2,3-triazole-4-carboxamides were synthesized via the condensation of variety of 1H-1,2,3-triazole-4-carboxylic acids and 4-thiocyanatoaniline using CDI as amide coupling reagents. According to computer-aided calculations, all synthesized compounds are expected to have acceptable ADME profile for drug design. The antiproliferative potency of derivatives was evaluated towards different cell lines. The specific activity of four N-(4-thiocyanatophenyl)-1H-1,2,3-triazole-4-carboxamides (4a, 4b, 4c, 4f) was comparable to doxorubicin (GI50 = 0.65 μM) at nanomolar level against Jurkat cells in the range of GI50 0.63–0.69 μM. According to the results of toxicity studies of the compounds for HEK293, HaCaT, Balb/c 3T3 cells, compound 4a was selected for further studies as a biocompatible agent with promising anticancer activity in the NCI60 cell lines. A remarkable antiproliferative activity of compound 4a towards leukemia cell lines (SR, MOLT-4; CCRF-CEM; HL-60(TB); K-562; RPMI-8226) was observed and high cytotoxicity towards the CAKI-1 (kidney cancer), LOX IMVI (melanoma) and UO-31 (renal cancer) cells lines was detected. Compound 4a inhibits LOX IMVI cells growth at a GI50 value of 0.15 μM. COMPARE analysis to indicate potential mechanisms of action of novel compound, as well as in silico SwissTargetPrediction and SwissSimilarity were performed. Compound 4a induced morphological changes (apoptotic bodies, membrane blebbing, chromatin condensation, and DNA fragmentation) in Jurkat T-cells. It reduced mitochondrial membrane potential and induced DNA damage in Jurkat cells without binding and/or intercalation to DNA molecule.
中文翻译:

新型 N-(4-thiocyanatophenyl)-1H-1,2,3-triazole-4-carboxamides 在纳摩尔剂量下对人类白血病 T 细胞表现出选择性细胞毒活性
N- ( 4-thiocyanatophenyl)-1 H- 1,2,3-triazole-4-carboxamides 由多种 1 H- 1,2,3-triazole-4-carboxy 酸和 4-thiocyanatoaniline缩合合成使用 CDI 作为酰胺偶联剂。根据计算机辅助计算,所有合成的化合物都有望具有可接受的药物设计 ADME 特征。评估了衍生物对不同细胞系的抗增殖效力。四种N- (4-thiocyanatophenyl)-1 H- 1,2,3-triazole-4-carboxamides ( 4a, 4b, 4c, 4f ) 的比活性在纳摩尔水平上与阿霉素 (GI 50 = 0.65 μM)相当针对 GI 50范围内的 Jurkat 细胞0.63–0.69 微米。根据化合物对 HEK293、HaCaT、Balb/c 3T3 细胞的毒性研究结果,选择化合物4a作为在 NCI60 细胞系中具有良好抗癌活性的生物相容剂进行进一步研究。观察到化合物4a对白血病细胞系(SR、MOLT-4;CCRF-CEM;HL-60(TB);K-562;RPMI-8226)具有显着的抗增殖活性,并且对 CAKI-1(肾癌)具有高细胞毒性)、LOX IMVI (黑色素瘤) 和 UO-31 (肾癌) 细胞系被检测到。化合物4a在 0.15 μM 的 GI 50值下抑制 LOX IMVI 细胞生长。比较分析以表明新化合物的潜在作用机制,以及在计算机上执行了 SwissTargetPrediction 和 SwissSimilarity。化合物4a在 Jurkat T 细胞中诱导了形态学变化(凋亡小体、膜起泡、染色质凝聚和 DNA 断裂)。它在不与 DNA 分子结合和/或嵌入的情况下降低了 Jurkat 细胞中的线粒体膜电位并诱导 DNA 损伤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号