Gastroenterology ( IF 25.7 ) Pub Date : 2022-08-06 , DOI: 10.1053/j.gastro.2022.07.052 Marie Truyens 1 , Triana Lobatón 2 , Marc Ferrante 3 , Peter Bossuyt 4 , Séverine Vermeire 3 , Lieven Pouillon 4 , Pieter Dewint 5 , Anneline Cremer 6 , Harald Peeters 7 , Guy Lambrecht 8 , Edouard Louis 9 , Jean-François Rahier 10 , Olivier Dewit 11 , Vinciane Muls 12 , Tom Holvoet 13 , Liv Vandermeulen 14 , Anneleen Peeters 15 , Gerard Bryan Gonzales 16 , Simon Bos 17 , Debby Laukens 17 , Martine De Vos 18
|
Background & Aims
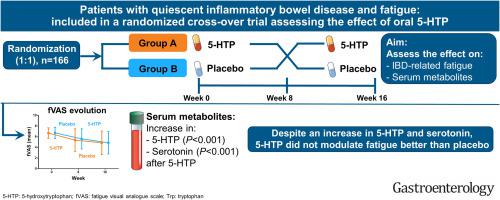
Fatigue is highly prevalent among patients with inflammatory bowel disease (IBD), and only limited treatment options are available. Based on the hypothetical link between low serum tryptophan concentrations and fatigue, we determined the effect of 5-hydroxytryptophan supplementation on fatigue in patients with inactive IBD.
Methods
A multicenter randomized controlled trial was performed at 13 Belgian hospitals, including 166 patients with IBD in remission but experiencing fatigue, defined by a fatigue visual analog scale (fVAS) score of ≥5. Patients were treated in a crossover manner with 100 mg oral 5-hydroxytryptophan or placebo twice daily for 2 consecutive periods of 8 weeks. The primary end point was the proportion of patients reaching a ≥20% reduction in fVAS after 8 weeks of intervention. Secondary outcomes included changes in serum tryptophan metabolites, Functional Assessment of Chronic Illness Therapy Fatigue scale, and scores for depression, anxiety, and stress. The effect of the intervention on the outcomes was evaluated by linear mixed modeling.
Results
During 5-hydroxytryptophan treatment, a significant increase in serum 5-hydroxytryptophan (estimated mean difference, 52.66 ng/mL; 95% confidence interval [CI], 39.34–65.98 ng/mL; P < .001) and serotonin (3.0 ng/mL; 95 CI, 1.97–4.03 ng/mL; P < .001) levels was observed compared with placebo. The proportion of patients reaching ≥20% reduction in fVAS was similar in placebo- (37.6%) and 5-hydroxytryptophan (35.6%)-treated patients (P = .830). The fVAS reduction (−0.18; 95% CI, −0.81 to 0.46; P = .581) and Functional Assessment of Chronic Illness Therapy Fatigue scale increase (0.68; 95% CI, −2.37 to 3.73; P = .660) were both comparable between 5-hydroxytryptophan and placebo treatment as well as changes in depression, anxiety, and stress scores.
Conclusions
Despite a significant increase in serum 5-hydroxytryptophan and serotonin levels, oral 5-hydroxytryptophan did not modulate IBD-related fatigue better than placebo. (Trial Registration: Belgian Federal Agency for Medication and Health Products, EudraCT number: 2017-005059-10 and ClinicalTrials.gov: NCT03574948, https://clinicaltrials.gov/ct2/show/NCT03574948.)
中文翻译:

5-羟基色氨酸对静止性炎症性肠病疲劳的影响:一项随机对照试验
背景与目标
疲劳在炎症性肠病 (IBD) 患者中非常普遍,并且只有有限的治疗选择可用。基于低血清色氨酸浓度与疲劳之间的假设联系,我们确定了补充 5-羟色氨酸对非活动性 IBD 患者疲劳的影响。
方法
在比利时的 13 家医院进行了一项多中心随机对照试验,其中包括 166 名 IBD 患者,这些患者处于缓解期但感到疲劳,其定义为疲劳视觉模拟量表 (fVAS) 评分≥5。患者以交叉方式接受 100 mg 口服 5-羟色氨酸或安慰剂治疗,每日两次,连续 2 次,为期 8 周。主要终点是干预 8 周后 fVAS 降低≥20% 的患者比例。次要结果包括血清色氨酸代谢物的变化、慢性疾病治疗疲劳量表的功能评估以及抑郁、焦虑和压力的评分。通过线性混合模型评估干预对结果的影响。
结果
在 5-羟色氨酸治疗期间,血清 5-羟色氨酸(估计平均差为 52.66 ng/mL;95% 置信区间 [CI],39.34–65.98 ng/mL;P < .001)和血清素(3.0 ng / mL mL;95 CI,1.97–4.03 ng/mL;P < .001) 与安慰剂相比观察到水平。fVAS 降低≥20% 的患者比例与安慰剂 (37.6%) 和 5-羟色氨酸 (35.6%) 治疗的患者相似 ( P = .830)。fVAS 降低(-0.18;95% CI,-0.81 至 0.46;P = .581)和慢性疾病治疗疲劳量表的功能评估增加(0.68;95% CI,-2.37 至 3.73;P = .660) 在 5-羟色氨酸和安慰剂治疗以及抑郁、焦虑和压力评分的变化之间具有可比性。
结论
尽管血清 5-羟色氨酸和血清素水平显着增加,但口服 5-羟色氨酸并没有比安慰剂更好地调节 IBD 相关疲劳。(试验注册:比利时联邦药物和健康产品署,EudraCT 编号:2017-005059-10 和 ClinicalTrials.gov:NCT03574948,https://clinicaltrials.gov/ct2/show/NCT03574948。)











































 京公网安备 11010802027423号
京公网安备 11010802027423号