当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Going Beyond the Local Catalytic Activity Space of Chitinase Using a Simulation-Based Iterative Saturation Mutagenesis Strategy
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-05 , DOI: 10.1021/acscatal.2c01466 Jinlong Li 1, 2 , Sijia Wang 1 , Cui Liu 1, 3 , Yixin Li 1 , Yu Wei 4 , Gang Fu 1 , Pi Liu 1, 3 , Hongwu Ma 1, 3 , Dawei Huang 5 , Jianping Lin 4 , Dawei Zhang 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-05 , DOI: 10.1021/acscatal.2c01466 Jinlong Li 1, 2 , Sijia Wang 1 , Cui Liu 1, 3 , Yixin Li 1 , Yu Wei 4 , Gang Fu 1 , Pi Liu 1, 3 , Hongwu Ma 1, 3 , Dawei Huang 5 , Jianping Lin 4 , Dawei Zhang 1, 2
Affiliation
|
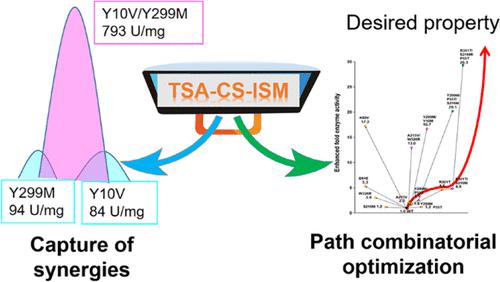
Many advances in modifying enzyme properties have been made based on the computation of stable transition states and experimental directed evolution. However, there are still great challenges related to difficult transition state (TS) calculations and the large number of evolutionary experiments needed for verification. The transition state analogue (TSA) has chemical similarity with the TS structure and can be used as a proxy for the unknown TS. Here, we proposed a computation-driven strategy to optimize structures of enzymes via simulation-based iterative saturation mutagenesis (ISM), aiming to stabilize the spatial effects of the TS with a TSA as a placeholder. This approach was applied to redesign structures of chitinase in order to increase its catalytic activity. After three rounds of simulation-based ISM, the catalytic activity of the triple mutant P55T/S216M/R301T was 29.3-fold higher than that of the wild type. The enrichment ratio of variants with increased activity reached 83% in the library selected for experimental verification. In this study, the strategy of optimizing enzymatic activity by computational ISM based on TSA has been streamlined, which facilitates the process of ISM and can be used to extend the bounds of the local optimal solution space.
中文翻译:

使用基于模拟的迭代饱和诱变策略超越几丁质酶的局部催化活性空间
基于稳定过渡态的计算和实验定向进化,在修饰酶特性方面取得了许多进展。然而,仍然存在与困难的过渡态(TS)计算和验证所需的大量进化实验相关的巨大挑战。过渡态类似物 (TSA) 与 TS 结构具有化学相似性,可用作未知 TS 的代理。在这里,我们提出了一种计算驱动的策略,通过基于模拟的迭代饱和诱变 (ISM) 优化酶的结构,旨在以 TSA 作为占位符来稳定 TS 的空间效应。该方法用于重新设计几丁质酶的结构,以提高其催化活性。经过三轮基于仿真的 ISM,三突变体P55T/S216M/R301T的催化活性比野生型高29.3倍。在选择进行实验验证的库中,活性增加的变异体的富集率达到了 83%。本研究简化了基于 TSA 的计算 ISM 优化酶活性的策略,这促进了 ISM 的过程,可用于扩展局部最优解空间的边界。
更新日期:2022-08-05
中文翻译:

使用基于模拟的迭代饱和诱变策略超越几丁质酶的局部催化活性空间
基于稳定过渡态的计算和实验定向进化,在修饰酶特性方面取得了许多进展。然而,仍然存在与困难的过渡态(TS)计算和验证所需的大量进化实验相关的巨大挑战。过渡态类似物 (TSA) 与 TS 结构具有化学相似性,可用作未知 TS 的代理。在这里,我们提出了一种计算驱动的策略,通过基于模拟的迭代饱和诱变 (ISM) 优化酶的结构,旨在以 TSA 作为占位符来稳定 TS 的空间效应。该方法用于重新设计几丁质酶的结构,以提高其催化活性。经过三轮基于仿真的 ISM,三突变体P55T/S216M/R301T的催化活性比野生型高29.3倍。在选择进行实验验证的库中,活性增加的变异体的富集率达到了 83%。本研究简化了基于 TSA 的计算 ISM 优化酶活性的策略,这促进了 ISM 的过程,可用于扩展局部最优解空间的边界。











































 京公网安备 11010802027423号
京公网安备 11010802027423号