当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modular Synthesis of Multifunctionalized CF3-Allenes through Selective Activation of Saturated Hydrocarbons
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-05 , DOI: 10.1021/acscatal.2c01521 Wenfeng Liu 1 , Chuhan Liu 1 , Minyan Wang 2 , Wangqing Kong 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-05 , DOI: 10.1021/acscatal.2c01521 Wenfeng Liu 1 , Chuhan Liu 1 , Minyan Wang 2 , Wangqing Kong 1
Affiliation
|
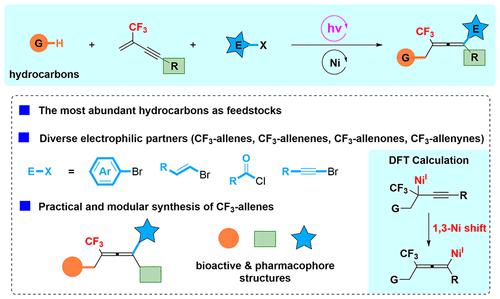
Catalytic 1,4-dicarbofunctionalization of 1,3-enynes is a powerful strategy for the synthesis of polysubstituted allenes. Despite impressive progress, such a strategy is still restricted to the use of alkyl-metallic reagents or pre-activated radical precursors, thus limiting its functional group compatibility and atom economy. Herein, we report that through the combination of decatungstate photo-hydrogen atom transfer and nickel catalysis, a three-component 1,4-dicarbofunctionalization of 2-trifluoromethyl-1,3-enynes is achieved. This strategy allows the modular synthesis of tetrasubstituted CF3-allenes under exceptionally mild conditions. A variety of electrophiles such as aryl bromides, alkenyl bromides, acyl chlorides, and alkynyl bromides were successfully employed as traps to lead to the desired products. Another significant advantage is that the most abundant hydrocarbons are used as feedstocks, and a wide range of synthetically versatile functional groups and complex drug-like structures can be easily incorporated. Based on experimental and density functional theories, a possible catalytic cycle involving 1,3-nickel rearrangement is proposed.
中文翻译:

通过饱和烃的选择性活化模块化合成多功能CF3-丙二烯
1,3-烯炔的催化 1,4-二碳官能化是合成多取代丙二烯的有力策略。尽管取得了令人瞩目的进展,但这种策略仍仅限于使用烷基金属试剂或预活化自由基前体,从而限制了其官能团兼容性和原子经济性。在此,我们报道通过十钨酸盐光氢原子转移和镍催化的结合,实现了 2-三氟甲基-1,3-烯炔的三组分 1,4-二碳官能化。该策略允许模块化合成四取代 CF 3-丙二烯在异常温和的条件下。多种亲电子试剂如芳基溴化物、烯基溴化物、酰氯和炔基溴化物被成功地用作捕集器以产生所需的产物。另一个显着优势是最丰富的碳氢化合物被用作原料,并且可以很容易地结合广泛的合成多功能官能团和复杂的药物样结构。基于实验和密度泛函理论,提出了一种可能的涉及 1,3-镍重排的催化循环。
更新日期:2022-08-05
中文翻译:

通过饱和烃的选择性活化模块化合成多功能CF3-丙二烯
1,3-烯炔的催化 1,4-二碳官能化是合成多取代丙二烯的有力策略。尽管取得了令人瞩目的进展,但这种策略仍仅限于使用烷基金属试剂或预活化自由基前体,从而限制了其官能团兼容性和原子经济性。在此,我们报道通过十钨酸盐光氢原子转移和镍催化的结合,实现了 2-三氟甲基-1,3-烯炔的三组分 1,4-二碳官能化。该策略允许模块化合成四取代 CF 3-丙二烯在异常温和的条件下。多种亲电子试剂如芳基溴化物、烯基溴化物、酰氯和炔基溴化物被成功地用作捕集器以产生所需的产物。另一个显着优势是最丰富的碳氢化合物被用作原料,并且可以很容易地结合广泛的合成多功能官能团和复杂的药物样结构。基于实验和密度泛函理论,提出了一种可能的涉及 1,3-镍重排的催化循环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号