当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Water Addition to Isopropanol for Hydrogenation of Compounds Derived from 5-Hydroxymethyl Furfural over Pd, Ru, and Cu Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-05 , DOI: 10.1021/acscatal.2c02445 Elise B. Gilcher 1 , Hochan Chang 1 , Michael Rebarchik 1 , George W. Huber 1 , James A. Dumesic 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-05 , DOI: 10.1021/acscatal.2c02445 Elise B. Gilcher 1 , Hochan Chang 1 , Michael Rebarchik 1 , George W. Huber 1 , James A. Dumesic 1
Affiliation
|
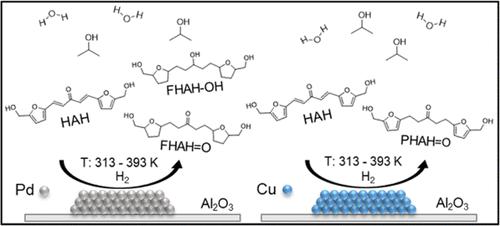
We have developed lumped reaction schemes to optimize the yields of products from selective hydrogenations of HAH, a biomass-derived platform chemical produced by two-step aldol condensations of 5-hydroxymethyl furfural (H) with acetone (A). Reaction schemes consisting of 7, 9, and 11 steps were examined to describe the rates of formation of the observed products and reaction intermediates for hydrogenation of HAH over Ru and Pd catalysts, and a 3-step scheme was studied over Cu catalysts. Rate constants and activation energies were calculated using these reaction schemes, and we then apply the schemes to explore the effects of water addition on the hydrogenation pathways. The effects of water addition to isopropanol (IPA) solvents on the hydrogenation of HAH were markedly different over Pd, Ru, and Cu catalysts. Over the Pd catalyst, the addition of water to IPA increased hydrogenation rates and promoted the hydrogenation of furan rings. The addition of water to IPA yielded significant carbon losses over the Ru catalyst, and slowed hydrogenation steps over Cu, while significantly inhibiting hydrogenation of the ketone group. This behavior opened routes toward increased production rates of PHAH═O (a partially hydrogenated, P, form of HAH containing a C═O bond), a product in which the diene groups of the furan rings were not hydrogenated. The addition of water also allowed increased feed concentrations of HAH that were previously not possible in pure IPA solvents. The insights presented in this work provide a more mechanistic description of the hydrogenation of HAH, the behavior of specific intermediates, and the reactivity of key functional groups.
中文翻译:

异丙醇中加水对 5-羟甲基糠醛化合物在 Pd、Ru 和 Cu 催化剂上加氢的影响
我们开发了集中反应方案来优化 HAH 的选择性氢化产物的产率,HAH 是一种生物质衍生的平台化学品,由 5-羟甲基糠醛 (H) 与丙酮 (A) 的两步羟醛缩合产生。研究了由 7、9 和 11 步组成的反应方案,以描述在 Ru 和 Pd 催化剂上 HAH 氢化的观察到的产物和反应中间体的形成速率,并在 Cu 催化剂上研究了 3 步方案。使用这些反应方案计算速率常数和活化能,然后我们应用这些方案来探索加水对氢化途径的影响。与 Pd、Ru 和 Cu 催化剂相比,异丙醇 (IPA) 溶剂中加水对 HAH 加氢的影响明显不同。在 Pd 催化剂上,向 IPA 中添加水提高了氢化率并促进了呋喃环的氢化。向 IPA 中添加水在 Ru 催化剂上产生了显着的碳损失,并减慢了 Cu 上的氢化步骤,同时显着抑制了酮基的氢化。这种行为开辟了提高 PHAH=O(含 C=O 键的部分氢化 P 形式的 HAH)产率的途径,其中呋喃环的二烯基团未氢化。添加水还可以提高 HAH 的进料浓度,这在以前在纯 IPA 溶剂中是不可能的。这项工作中提出的见解为 HAH 的氢化、特定中间体的行为和关键官能团的反应性提供了更机械的描述。
更新日期:2022-08-05
中文翻译:

异丙醇中加水对 5-羟甲基糠醛化合物在 Pd、Ru 和 Cu 催化剂上加氢的影响
我们开发了集中反应方案来优化 HAH 的选择性氢化产物的产率,HAH 是一种生物质衍生的平台化学品,由 5-羟甲基糠醛 (H) 与丙酮 (A) 的两步羟醛缩合产生。研究了由 7、9 和 11 步组成的反应方案,以描述在 Ru 和 Pd 催化剂上 HAH 氢化的观察到的产物和反应中间体的形成速率,并在 Cu 催化剂上研究了 3 步方案。使用这些反应方案计算速率常数和活化能,然后我们应用这些方案来探索加水对氢化途径的影响。与 Pd、Ru 和 Cu 催化剂相比,异丙醇 (IPA) 溶剂中加水对 HAH 加氢的影响明显不同。在 Pd 催化剂上,向 IPA 中添加水提高了氢化率并促进了呋喃环的氢化。向 IPA 中添加水在 Ru 催化剂上产生了显着的碳损失,并减慢了 Cu 上的氢化步骤,同时显着抑制了酮基的氢化。这种行为开辟了提高 PHAH=O(含 C=O 键的部分氢化 P 形式的 HAH)产率的途径,其中呋喃环的二烯基团未氢化。添加水还可以提高 HAH 的进料浓度,这在以前在纯 IPA 溶剂中是不可能的。这项工作中提出的见解为 HAH 的氢化、特定中间体的行为和关键官能团的反应性提供了更机械的描述。










































 京公网安备 11010802027423号
京公网安备 11010802027423号