当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiol Reactivity of N-Aryl α-Methylene-γ-lactams: Influence of the Guaianolide Structure
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.joc.2c01530 Daniel P Dempe 1 , Chong-Lei Ji , Peng Liu 1, 2 , Kay M Brummond 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.joc.2c01530 Daniel P Dempe 1 , Chong-Lei Ji , Peng Liu 1, 2 , Kay M Brummond 1
Affiliation
|
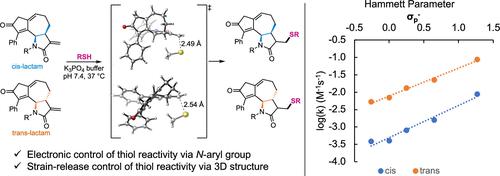
The α-methylene-γ-lactam offers promise as a complementary warhead for the development of targeted covalent inhibitors. However, an understanding of the factors governing its electrophilic reactivity is needed to promote the development of lead compounds utilizing this motif. Herein we synthesize a series of N-aryl-substituted α-methylene-γ-lactams installed within the framework of a bioactive guaianolide analog. To determine the effects of the guaianolide structure on the electrophilic reactivity, these compounds were reacted with glutathione under biomimetic conditions, and the rate constants were measured. A linear free-energy relationship was observed with the Hammett parameter of the N-aryl group within the cis- or trans-annulated isomeric series of compounds. However, the trans-annulated compounds exhibited a ca. 10-fold increase in reactivity relative to both the cis-annulated compounds and the corresponding N-arylated 3-methylene-2-pyrrolidinones. Density functional theory calculations revealed that the reactivity of the trans-annulated stereoisomers is promoted by the partial release of the ring strain of the fused seven-membered ring in the thio-Michael addition transition state.
中文翻译:

N-芳基α-亚甲基-γ-内酰胺的硫醇反应性:愈创木脂结构的影响
α-亚甲基-γ-内酰胺有望成为开发靶向共价抑制剂的补充弹头。然而,需要了解控制其亲电反应性的因素,以促进利用该基序的先导化合物的开发。在这里,我们合成了一系列N-芳基取代的 α-亚甲基-γ-内酰胺,安装在生物活性愈创木内酯类似物的框架内。为了确定愈创木酚内酯结构对亲电反应性的影响,这些化合物在仿生条件下与谷胱甘肽反应,并测量了速率常数。顺式或反式内N-芳基的 Hammett 参数观察到线性自由能关系-环状异构系列化合物。然而,反式环状化合物表现出约。相对于顺式环化化合物和相应的N-芳基化 3-亚甲基-2-吡咯烷酮,反应性提高了 10 倍。密度泛函理论计算表明,硫代迈克尔加成过渡态稠合七元环的环应变的部分释放促进了反式环化立体异构体的反应性。
更新日期:2022-08-05
中文翻译:

N-芳基α-亚甲基-γ-内酰胺的硫醇反应性:愈创木脂结构的影响
α-亚甲基-γ-内酰胺有望成为开发靶向共价抑制剂的补充弹头。然而,需要了解控制其亲电反应性的因素,以促进利用该基序的先导化合物的开发。在这里,我们合成了一系列N-芳基取代的 α-亚甲基-γ-内酰胺,安装在生物活性愈创木内酯类似物的框架内。为了确定愈创木酚内酯结构对亲电反应性的影响,这些化合物在仿生条件下与谷胱甘肽反应,并测量了速率常数。顺式或反式内N-芳基的 Hammett 参数观察到线性自由能关系-环状异构系列化合物。然而,反式环状化合物表现出约。相对于顺式环化化合物和相应的N-芳基化 3-亚甲基-2-吡咯烷酮,反应性提高了 10 倍。密度泛函理论计算表明,硫代迈克尔加成过渡态稠合七元环的环应变的部分释放促进了反式环化立体异构体的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号