当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of Chlorine Dioxide with Saturated Nitrogen-Containing Heterocycles and Comparison with the Micropollutant Behavior in a Real Water Matrix
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.est.1c08381 Mohammad Sajjad Abdighahroudi 1, 2 , Xenia A M Mutke 1 , Mischa Jütte 1, 2 , Katharina Klein 1 , Torsten C Schmidt 1, 3, 4 , Holger V Lutze 1, 2, 3, 4
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.est.1c08381 Mohammad Sajjad Abdighahroudi 1, 2 , Xenia A M Mutke 1 , Mischa Jütte 1, 2 , Katharina Klein 1 , Torsten C Schmidt 1, 3, 4 , Holger V Lutze 1, 2, 3, 4
Affiliation
|
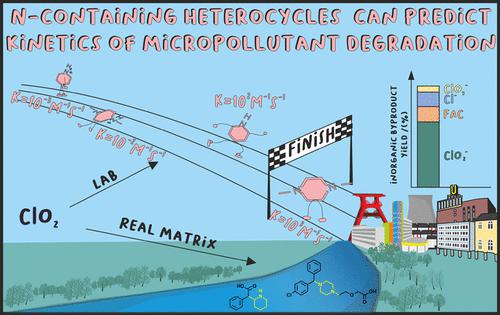
Chlorine dioxide (ClO2) is a very selective oxidant that reacts with electron-rich moieties such as activated amines and thus can degrade specific N-containing micropollutants. N-containing heterocycles (NCHs) are among the most frequent moieties of pharmaceuticals. In this study, the reactions of ClO2 with ritalinic acid and cetirizine, two abundant micropollutants, and model compounds representing their NCH moiety were investigated. The pH-dependent apparent reaction rates of all NCHs with ClO2 were measured and modeled. This model showed that neutral amines are the most important species having reaction rates between 800 and 3200 M–1 s–1, while cationic amines are not reactive. Ritalinic acid, cetirizine, and their representative model compounds showed a high stoichiometric ratio of ≈5 moles ClO2 consumption per degraded ritalinic acid and ≈4 moles ClO2 consumption per degraded cetirizine, respectively. Investigation of chlorine-containing byproducts of ClO2 showed that all investigated NCHs mostly react by electron transfer and form above 80% chlorite. The reactions of the model compounds were well comparable with cetirizine and ritalinic acid, indicating that the model compounds indeed represented the reaction centers of cetirizine and ritalinic acid. Using the calculated apparent reaction rate constants, micropollutant degradation during ClO2 treatment of surface water was predicted for ritalinic acid and cetirizine with −8 to −15% and 13 to −22% error, respectively. The results indicate that in ClO2-based treatment, piperidine-containing micropollutants such as ritalinic acid can be considered not degradable, while piperazine-containing compounds such as cetirizine can be moderately degraded. This shows that NCH model compounds could be used to predict micropollutant degradation.
中文翻译:

二氧化氯与饱和含氮杂环的反应及与真实水基质中微污染物行为的比较
二氧化氯 (ClO 2 ) 是一种选择性很强的氧化剂,它与富含电子的部分(如活化胺)反应,因此可以降解特定的含氮微污染物。含氮杂环 ( NCH ) 是最常见的药物部分之一。在这项研究中,研究了 ClO 2与利他林酸和西替利嗪这两种丰富的微污染物以及代表其 NCH 部分的模型化合物的反应。测量和模拟了所有 NCH 与 ClO 2的 pH 依赖性表观反应速率。该模型表明,中性胺是最重要的物质,反应速率在 800 和 3200 M –1 s –1之间,而阳离子胺不具有反应性。利他林酸、西替利嗪及其代表模型化合物显示出高化学计量比,每个降解的利他利酸消耗 ≈5 摩尔 ClO 2消耗,每个降解的西替利嗪消耗 ≈4 摩尔 ClO 2消耗,分别。对 ClO 2的含氯副产物的研究表明,所有研究的 NCH 主要通过电子转移反应并形成 80% 以上的亚氯酸盐。模型化合物的反应与西替利嗪和利他林酸的反应具有很好的可比性,表明模型化合物确实代表了西替利嗪和利他林酸的反应中心。使用计算的表观反应速率常数,ClO 2过程中的微污染物降解预测利他林酸和西替利嗪处理地表水的误差分别为 -8 至 -15% 和 13 至 -22%。结果表明,在基于ClO 2的处理中,含哌啶的微污染物如利他林酸可被认为是不可降解的,而含哌嗪的化合物如西替利嗪可被适度降解。这表明 NCH 模型化合物可用于预测微污染物降解。
更新日期:2022-08-05
中文翻译:

二氧化氯与饱和含氮杂环的反应及与真实水基质中微污染物行为的比较
二氧化氯 (ClO 2 ) 是一种选择性很强的氧化剂,它与富含电子的部分(如活化胺)反应,因此可以降解特定的含氮微污染物。含氮杂环 ( NCH ) 是最常见的药物部分之一。在这项研究中,研究了 ClO 2与利他林酸和西替利嗪这两种丰富的微污染物以及代表其 NCH 部分的模型化合物的反应。测量和模拟了所有 NCH 与 ClO 2的 pH 依赖性表观反应速率。该模型表明,中性胺是最重要的物质,反应速率在 800 和 3200 M –1 s –1之间,而阳离子胺不具有反应性。利他林酸、西替利嗪及其代表模型化合物显示出高化学计量比,每个降解的利他利酸消耗 ≈5 摩尔 ClO 2消耗,每个降解的西替利嗪消耗 ≈4 摩尔 ClO 2消耗,分别。对 ClO 2的含氯副产物的研究表明,所有研究的 NCH 主要通过电子转移反应并形成 80% 以上的亚氯酸盐。模型化合物的反应与西替利嗪和利他林酸的反应具有很好的可比性,表明模型化合物确实代表了西替利嗪和利他林酸的反应中心。使用计算的表观反应速率常数,ClO 2过程中的微污染物降解预测利他林酸和西替利嗪处理地表水的误差分别为 -8 至 -15% 和 13 至 -22%。结果表明,在基于ClO 2的处理中,含哌啶的微污染物如利他林酸可被认为是不可降解的,而含哌嗪的化合物如西替利嗪可被适度降解。这表明 NCH 模型化合物可用于预测微污染物降解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号