当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hypervalent Iodine Reagents Enable C–H Alkynylation with Iminophenylacetic Acids via Alkoxyl Radicals
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.orglett.2c02210 Zhengyi Liu 1 , Yue Pan 1, 2 , Peng Zou 1 , Hanchu Huang 1 , Yali Chen 2 , Yiyun Chen 1, 3, 4
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.orglett.2c02210 Zhengyi Liu 1 , Yue Pan 1, 2 , Peng Zou 1 , Hanchu Huang 1 , Yali Chen 2 , Yiyun Chen 1, 3, 4
Affiliation
|
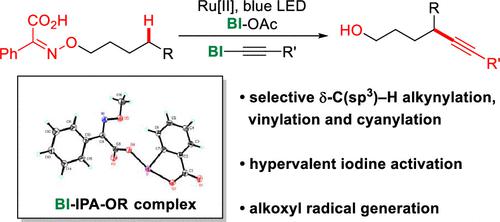
Here we report δ-C–H alkynylation to synthesize various δ-alkynols from iminophenylacetic acids. The hypervalent iodine-coordinated benziodoxole-alkoxyl-iminophenylacetic acid complex was the key intermediate and was characterized by X-ray crystallography for the first time. δ-C–H alkynylation is compatible with sensitive functional groups, including azides, aldehydes, and free alcohols, for the synthesis of δ-alkynols with diversified substituents in excellent regioselectivity. This reaction extends to δ-hydroxylalkene and δ-hydroxylnitrile synthesis, and the δ-alkynol products are easily derivatized to other valuable bifunctional building blocks.
中文翻译:

高价碘试剂通过烷氧基自由基与亚氨基苯乙酸实现 C-H 炔基化
在这里,我们报告了 δ-C-H 炔基化以从亚氨基苯乙酸合成各种 δ-炔醇。高价碘配位的苯并氧唑-烷氧基-亚氨基苯乙酸络合物是关键中间体,首次通过X射线晶体学对其进行了表征。δ-C-H 炔基化与敏感官能团相容,包括叠氮化物、醛和游离醇,用于合成具有优异区域选择性的多种取代基的 δ-炔醇。该反应扩展到 δ-羟基烯烃和 δ-羟基腈的合成,δ-炔醇产物很容易衍生为其他有价值的双功能结构单元。
更新日期:2022-08-05
中文翻译:

高价碘试剂通过烷氧基自由基与亚氨基苯乙酸实现 C-H 炔基化
在这里,我们报告了 δ-C-H 炔基化以从亚氨基苯乙酸合成各种 δ-炔醇。高价碘配位的苯并氧唑-烷氧基-亚氨基苯乙酸络合物是关键中间体,首次通过X射线晶体学对其进行了表征。δ-C-H 炔基化与敏感官能团相容,包括叠氮化物、醛和游离醇,用于合成具有优异区域选择性的多种取代基的 δ-炔醇。该反应扩展到 δ-羟基烯烃和 δ-羟基腈的合成,δ-炔醇产物很容易衍生为其他有价值的双功能结构单元。











































 京公网安备 11010802027423号
京公网安备 11010802027423号