当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Driven [3 + 2]/[4 + 2] Annulation Reactions of Alkenes with N-Aminopyridinium Salts
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.orglett.2c02323 Yu-Zhao Wang 1 , Peng-Yu Liang 1 , Hong-Chao Liu 1 , Wu-Jie Lin 1 , Pan-Pan Zhou 1 , Wei Yu 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.orglett.2c02323 Yu-Zhao Wang 1 , Peng-Yu Liang 1 , Hong-Chao Liu 1 , Wu-Jie Lin 1 , Pan-Pan Zhou 1 , Wei Yu 1
Affiliation
|
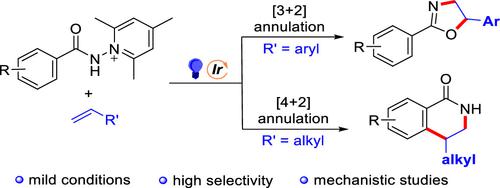
The annulation reactions of benzoamidyl radicals with alkenes were realized under visible light irradiation with fac-Ir(ppy)3 as catalyst and N-aminopyridinium salts as benzoamidyl radical precursors. The reaction can deliver two distinct types of products: in the case of vinyl arenes, [3 + 2] annulation product dihydrooxazoles were yielded exclusively; when alkyl-substituted alkenes were used, on the other hand, it afforded [4 + 2] annulation product dihydroisoquinolinones. Factors determining the reaction consequence were elucidated by DFT calculations.
中文翻译:

烯烃与 N-氨基吡啶鎓盐的可见光驱动 [3 + 2]/[4 + 2] 环化反应
以fac -Ir(ppy) 3为催化剂,N-氨基吡啶鎓盐为苯甲酰胺自由基前体,在可见光照射下实现了苯甲酰胺自由基与烯烃的环化反应。该反应可以产生两种不同类型的产物:在乙烯基芳烃的情况下,仅产生 [3 + 2] 环化产物二氢恶唑;另一方面,当使用烷基取代的烯烃时,它提供了[4 + 2]环化产物二氢异喹啉酮。通过 DFT 计算阐明了决定反应后果的因素。
更新日期:2022-08-05
中文翻译:

烯烃与 N-氨基吡啶鎓盐的可见光驱动 [3 + 2]/[4 + 2] 环化反应
以fac -Ir(ppy) 3为催化剂,N-氨基吡啶鎓盐为苯甲酰胺自由基前体,在可见光照射下实现了苯甲酰胺自由基与烯烃的环化反应。该反应可以产生两种不同类型的产物:在乙烯基芳烃的情况下,仅产生 [3 + 2] 环化产物二氢恶唑;另一方面,当使用烷基取代的烯烃时,它提供了[4 + 2]环化产物二氢异喹啉酮。通过 DFT 计算阐明了决定反应后果的因素。









































 京公网安备 11010802027423号
京公网安备 11010802027423号