Journal of Controlled Release ( IF 10.5 ) Pub Date : 2022-08-04 , DOI: 10.1016/j.jconrel.2022.07.033 Lei Dou 1 , Huiqin Liu 1 , Kaixin Wang 1 , Jing Liu 2 , Lei Liu 1 , Junxiao Ye 1 , Rui Wang 1 , Haiteng Deng 2 , Feng Qian 1
|
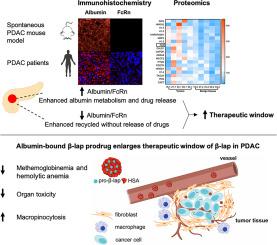
NAD(P)H:quinone oxidoreductase 1 (NQO1) is an enzyme significantly overexpressed in pancreatic ductal adenocarcinoma (PDAC) tumors compared to the associated normal tissues. NQO1 bioactivatable drugs, such as β-lapachone (β-lap), can be catalyzed to generate reactive oxygen species (ROS) for direct tumor killing. However, the extremely narrow therapeutic window caused by methemoglobinemia and hemolytic anemia severely restricts its further clinical translation despite considerable efforts in the past 20 years. Previously, we demonstrated that albumin could be utilized to deliver cytotoxic drugs selectively into KRAS-mutant PDAC with a much expanded therapeutic window due to KRAS-enhanced macropinocytosis and reduced neonatal Fc receptor (FcRn) expression in PDAC. Herein, we analyzed the expression patterns of albumin and FcRn across major organs in LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) mice. The tumors were the predominant tissues with both elevated albumin and reduced FcRn expression, thus making them an ideal target for albumin-based drug delivery. Quantitative proteomics analysis of tissue samples from 5 human PDAC patients further confirmed the elevated albumin/FcRn ratio. Given such a compelling biological rationale, we designed a nanoparticle albumin-bound prodrug of β-lap, nab-(pro-β-lap), to achieve PDAC targeted delivery and expand the therapeutic window of β-lap. We found that nab-(pro-β-lap) uptake was profoundly enhanced by KRAS mutation. Compared to the solution formulation of the parent drug β-lap, nab-(pro-β-lap) showed enhanced safety due to much lower rates of methemoglobinemia and hemolytic anemia, which was confirmed both in vitro and in vivo. Furthermore, nab-(pro-β-lap) significantly inhibited tumor growth in subcutaneously implanted KPC xenografts and enhanced the pharmacodynamic endpoints (e.g., PARP1 hyperactivation, γ-H2AX). Thus, nab-(pro-β-lap), with improved safety and antitumor efficacy, offers a drug delivery strategy with translational viability for β-lap in pancreatic cancer therapy.
中文翻译:

白蛋白结合使 NQO1 生物激活药物成为胰腺癌的新型疗法
NAD( P )H:醌氧化还原酶 1 (NQO1) 是一种在胰腺导管腺癌 (PDAC) 肿瘤中比相关正常组织显着过度表达的酶。 NQO1生物激活药物,例如β-拉帕酮(β-lap),可以被催化产生活性氧(ROS)以直接杀死肿瘤。然而,尽管在过去的20年中做出了巨大的努力,但高铁血红蛋白血症和溶血性贫血引起的极其狭窄的治疗窗严重限制了其进一步的临床转化。此前,我们证明白蛋白可用于选择性地将细胞毒性药物递送至KRAS突变型 PDAC,由于KRAS增强的巨胞饮作用和 PDAC 中新生儿 Fc 受体 (FcRn) 表达的减少,治疗窗口大大扩大。在此,我们分析了LSL-Kras G12D/+ ;LSL-Trp53 R172H/+ ;Pdx-1-Cre (KPC) 小鼠主要器官中白蛋白和 FcRn 的表达模式。肿瘤是白蛋白升高和 FcRn 表达降低的主要组织,因此使其成为基于白蛋白的药物递送的理想靶标。对 5 名人类 PDAC 患者组织样本的定量蛋白质组学分析进一步证实了白蛋白/FcRn 比率升高。鉴于如此令人信服的生物学原理,我们设计了一种纳米颗粒白蛋白结合的 β-lap 前药 nab-(pro-β-lap),以实现 PDAC 靶向递送并扩大 β-lap 的治疗窗口。我们发现KRAS突变极大地增强了 nab-(pro-β-lap)的摄取。 与母药 β-lap 的溶液制剂相比,nab-(pro-β-lap)的安全性更高,因为高铁血红蛋白血症和溶血性贫血的发生率大大降低,这一点在体外和体内均得到证实。此外,nab-(pro-β-lap) 显着抑制皮下植入的 KPC 异种移植物中的肿瘤生长,并增强药效学终点(例如 PARP1 过度激活、γ-H 2 AX)。因此,nab-(pro-β-lap) 具有更高的安全性和抗肿瘤功效,为胰腺癌治疗中的 β-lap 提供了一种具有转化活力的药物递送策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号