European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2022-08-05 , DOI: 10.1016/j.ejmech.2022.114651 Meng-Xue Wei 1 , Yi-Xuan Zhou 2 , Mengxia Lin 3 , Jun Zhang 2 , Xuanrong Sun 3
|
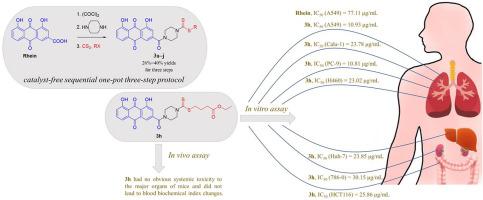
A series of novel rhein-piperazine-dithiocarbamate hybrids 3 were efficiently synthesized from rhein through a catalyst-free and one-pot, three-step sequence involving chlorination and N-acylation followed by dithiocarbamate formation. Hybrids 3 were evaluated for their in vitro cytotoxic potency by MTT assay against several human cancer and non-cancer cells. Five of the hybrids were more cytotoxic to human lung cancer cell line A549 than the parent rhein and the reference, cytarabine (CAR). Structure-activity relationship (SAR) analysis indicated that cytotoxicity was significantly enhanced when ester groups were incorporated into the hybrids (3h–j). In particular, hybrid 3h (IC50 = 10.93 μg/mL), containing a long-chain alkyl ester, was the most potent compound toward A549 tumor cells, being 7- and 5-fold more toxic than rhein (IC50 = 77.11 μg/mL) and CAR (IC50 = 49.27 μg/mL), respectively. Additionally, hybrid 3h was less toxic to the corresponding normal human lung fibroblast cell line, WI-38, with a higher selectivity index (SI, WI-38/A549 ≈ 5) than doxorubicin (DOX, SI ≈ 0), CAR (SI ≈ 2) and rhein (SI ≈ 1). Furthermore, hybrid 3h displayed more toxicity against four types of lung cancer cells (A549, Calu-1, PC-9, and H460; IC50 = 10.81–23.78 μg/mL) than against six other types of cancer cells (Huh-7, 786-O, HCT116, Hela, SK-BR-3, and SK-OV-3; IC50 = 23.85–51.98 μg/mL). Further mechanistic studies showed that hybrid 3h induced apoptosis in a concentration-dependent manner in human lung adenocarcinoma cell line PC-9. In vivo safety studies showed that hybrid 3h had no acute toxicity to the major organs of mice and did not lead to blood biochemical index changes. Our results exhibit prominent anti-cancer cell inhibition ability and no obvious systemic toxicity to normal organs, indicating that hybrid 3h has promising potential for further applications in anti-lung cancer drug development.
中文翻译:

大黄酸-哌嗪-二硫代氨基甲酸酯杂化物作为潜在抗癌剂的设计、合成和生物学评价
一系列新的大黄酸-哌嗪-二硫代氨基甲酸酯杂化物3是由大黄酸通过无催化剂和一锅法、三步序列(包括氯化和N-酰化,然后形成二硫代氨基甲酸酯)有效合成的。通过 MTT 测定法评估杂交体3针对几种人类癌症和非癌细胞的体外细胞毒性效力。其中五个杂种对人肺癌细胞系 A549 的细胞毒性比亲本大黄酸和参考阿糖胞苷 (CAR) 更强。构效关系(SAR)分析表明,当酯基掺入杂合体中时,细胞毒性显着增强(3h - j)。特别是混合含有长链烷基酯的3h (IC 50 = 10.93 μg/mL) 是对 A549 肿瘤细胞最有效的化合物,其毒性是大黄酸 (IC 50 = 77.11 μg/mL) 的 7 倍和 5 倍,并且CAR (IC 50 = 49.27 μg/mL)。此外,混合3h对相应的正常人肺成纤维细胞系 WI-38 的毒性较小,其选择性指数 (SI, WI-38/A549 ≈ 5) 高于阿霉素 (DOX, SI ≈ 0), CAR (SI ≈ 2) 和大黄酸 (SI ≈ 1)。此外,混合3h对四种类型的肺癌细胞(A549、Calu-1、PC-9 和 H460;IC 50 = 10.81–23.78 μg/mL),而不是针对六种其他类型的癌细胞(Huh-7、786-O、HCT116、Hela、SK-BR-3 和 SK-OV-3;IC 50 = 23.85–51.98 μg/毫升)。进一步的机制研究表明,杂交3h在人肺腺癌细胞系 PC-9 中以浓度依赖性方式诱导细胞凋亡。体内安全性研究表明,hybrid 3h对小鼠主要器官没有急性毒性,也不会导致血液生化指标的变化。我们的研究结果显示出显着的抗癌细胞抑制能力,对正常器官没有明显的全身毒性,表明混合3h在抗肺癌药物开发中具有进一步应用的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号