European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-08-05 , DOI: 10.1016/j.ejmech.2022.114632 Manuel K Langer 1 , Ataur Rahman 2 , Hymonti Dey 2 , Trude Anderssen 3 , Francesco Zilioli 1 , Tor Haug 2 , Hans-Matti Blencke 2 , Klara Stensvåg 2 , Morten B Strøm 3 , Annette Bayer 1
|
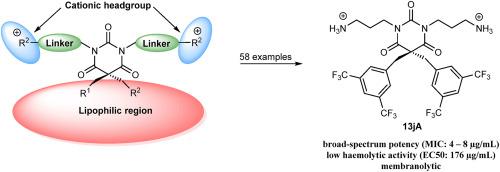
An amphipathic barbiturate mimic of the marine eusynstyelamides is reported as a promising class of antimicrobial agents. We hereby report a detailed analysis of the structure-activity relationship for cationic amphipathic N,N′-dialkylated-5,5-disubstituted barbiturates. The influence of various cationic groups, hydrocarbon linkers and lipophilic side chains on the compounds’ antimicrobial potency and haemolytic activity was studied. A comprehensive library of 58 compounds was prepared using a concise synthetic strategy. We found cationic amine and guanidyl groups to yield the highest broad-spectrum activity and cationic trimethylated quaternary amine groups to exert narrow-spectrum activity against Gram-positive bacteria. n-Propyl hydrocarbon linkers proved to be the best compromise between potency and haemolytic activity. The combination of two different lipophilic side chains allowed for further fine-tuning of the biological properties. Using these insights, we were able to prepare both, the potent narrow-spectrum barbiturate 8a and the broad-spectrum barbiturates 11lG and 20jG, all having low or no haemolytic activity. The guanidine derivative 11lG demonstrated a strong membrane disrupting effect in luciferase-based assays. We believe that these results may be valuable in further development of antimicrobial lead structures.
中文翻译:

抗菌阳离子两亲性巴比妥酸盐的简明 SAR 分析,以改善活性 - 毒性曲线
据报道,一种海洋eusynstylamides 的两亲性巴比妥酸盐模拟物是一类有前途的抗菌剂。我们在此报告了对阳离子两亲N,N'-二烷基化-5,5-二取代巴比妥酸盐的构效关系的详细分析。研究了各种阳离子基团、烃类接头和亲脂性侧链对化合物的抗微生物效力和溶血活性的影响。使用简洁的合成策略制备了一个包含 58 种化合物的综合库。我们发现阳离子胺和胍基产生最高的广谱活性,阳离子三甲基化季胺基对革兰氏阳性菌发挥窄谱活性。n-丙基烃接头被证明是效力和溶血活性之间的最佳折衷。两种不同的亲脂性侧链的组合允许进一步微调生物学特性。利用这些见解,我们能够制备强效窄谱巴比妥酸盐8a和广谱巴比妥酸盐11lG和20jG,它们都具有低溶血活性或没有溶血活性。胍衍生物11lG在基于荧光素酶的测定中表现出强烈的膜破坏作用。我们相信这些结果可能对进一步开发抗菌先导结构有价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号