当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of Propylene Epoxidation over Extracrystalline Gold Active Sites on AU/TS-1 Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-04 , DOI: 10.1021/acscatal.2c02213 Jeremy W. Arvay 1 , Wei Hong 2 , Christina Li 2 , W. Nicholas Delgass 1 , Fabio H. Ribeiro 1 , James W. Harris 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-04 , DOI: 10.1021/acscatal.2c02213 Jeremy W. Arvay 1 , Wei Hong 2 , Christina Li 2 , W. Nicholas Delgass 1 , Fabio H. Ribeiro 1 , James W. Harris 3
Affiliation
|
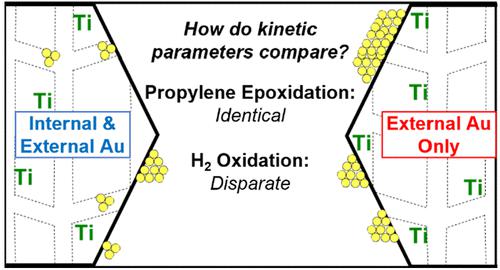
To determine whether the catalytic roles of extracrystalline and intracrystalline gold nanoparticles supported on titanosilicate-1 (TS-1) for direct propylene epoxidation are intrinsically different, the kinetics of direct propylene epoxidation were measured in a gas-phase continuous stirred tank reactor (CSTR) over polyvinylpyrrolidone-coated (PVP) gold nanoparticles (Au-PVP/TS-1) deposited on TS-1. The as-made PVP-coated gold nanoparticles were too large to fit into the micropores of TS-1, even after ligands were removed in situ by a series of pretreatments, as confirmed by both TEM and TGA-DSC. The activation energy (51 kJ mol–1) and reaction orders for H2 (1.3), O2 (0.4), propylene (0.4), propylene oxide (−0.6), carbon dioxide (0), and water (0) for propylene epoxidation measured on Au-PVP/TS-1 were consistent with those reported for Au/TS-1 prepared via deposition-precipitation (Au-DP/TS-1) (52 kJ mol–1, H2, 1; O2, 0.4; C3H6, 0.4; C3H6O, −0.6; CO2, 0; H2O, 0). However, while the reaction orders for hydrogen oxidation on Au-PVP/TS-1 (H2, 0.8; O2, 0; C3H6, −0.3; C3H6O, −0.1; CO2, 0; H2O, −0.2) were similar to those measured on Au-DP/TS-1 (H2, 0.9; O2, 0.3; C3H6, −0.3; C3H6O, 0; CO2, 0; H2O, −0.1), a decrease in activation energy from approximately 30 kJ mol–1 for Au-DP/TS-1 to 4–5 kJ mol–1 for Au-PVP/TS-1 suggests there is a change in rate-limiting step or active site for hydrogen oxidation. Additionally, an active site model was developed which determines the number of Ti within an interaction range of the perimeter of extracrystalline Au nanoparticles (i.e., the number of Au–Ti active site pairs). This model was used to estimate catalytic turnover frequencies over solely proximal Au–Ti pairs, assuming that hydrogen peroxide does not desorb and migrate from Au sites to Ti sites and instead propylene oxide forms in a concerted mechanism previously termed the “simultaneous” mechanism. Turnover frequencies estimated for this active site model for a data set containing both Au-DP/TS-1 and Au-PVP/TS-1 were ∼20× higher than the highest previous reported estimates (∼80 s–1 vs 1–5 s–1 at 473 K) for catalytic oxidation on noble metals, suggesting that the simultaneous mechanism occurring over proximal Au–Ti sites alone is incapable of explaining the observed rate of propylene epoxidation and that short-range migration of hydrogen peroxide is kinetically relevant. The agreement of reaction orders, activation energy, and active site model for propylene epoxidation on both Au-DP/TS-1 and Au-PVP/TS-1 suggests a common mechanism for propylene epoxidation on both catalysts containing small intraporous gold clusters and catalysts with exclusively larger extracrystalline gold nanoparticles. Rates of hydrogen oxidation were found to vary proportionally to the amount of surface gold atoms. This is also consistent with the hypothesis that the observed decrease in hydrogen efficiency and PO site-time-yield per gold mass with increasing gold loading are driven primarily by the gold dispersion in Au/TS-1 catalysts.
中文翻译:

AU/TS-1催化剂上晶外金活性位点的丙烯环氧化动力学
为了确定负载在钛硅酸盐-1 (TS-1) 上的晶外和晶内金纳米粒子对直接丙烯环氧化的催化作用是否存在本质上的不同,在气相连续搅拌釜反应器 (CSTR) 中测量了直接丙烯环氧化的动力学。在沉积在 TS-1 上的聚乙烯吡咯烷酮涂层 (PVP) 金纳米粒子 (Au-PVP/TS-1) 上。正如TEM 和 TGA-DSC 所证实的那样,即使在通过一系列预处理原位去除配体后,制成的 PVP 涂层金纳米颗粒也太大而无法装入 TS-1 的微孔中。H2 (1.3)、O 2的活化能 (51 kJ mol –1 ) 和反应级数(0.4)、丙烯 (0.4)、环氧丙烷 (-0.6)、二氧化碳 (0) 和水 (0) 用于在 Au-PVP/TS-1 上测量的丙烯环氧化与报告的 Au/TS-1 一致通过沉积-沉淀法制备 (Au-DP/TS-1) (52 kJ mol –1 , H 2 , 1; O 2 , 0.4; C 3 H 6 , 0.4; C 3 H 6 O, -0.6; CO 2 , 0;H 2 O,0)。然而,虽然 Au-PVP/TS-1 上的氢氧化反应顺序 (H 2 , 0.8; O 2 , 0; C 3 H 6 , -0.3; C 3 H 6 O, -0.1; CO 2 , 0; H2 O, -0.2) 与在 Au-DP/TS-1 上测量的相似 (H 2 , 0.9; O 2 , 0.3; C 3 H 6 , -0.3; C 3 H 6 O, 0; CO 2 , 0 ; H 2 O, -0.1),活化能从 Au-DP/TS-1 的大约 30 kJ mol –1降低到 4–5 kJ mol –1Au-PVP/TS-1 表明限速步骤或氢氧化的活性位点发生了变化。此外,还开发了一个活性位点模型,该模型确定了晶外 Au 纳米颗粒周边相互作用范围内的 Ti 数量(即,Au-Ti 活性位点对的数量)。该模型用于估计仅近端 Au-Ti 对上的催化转换频率,假设过氧化氢不会从 Au 位点解吸和迁移到 Ti 位点,而是以先前称为“同时”机制的协同机制形成环氧丙烷。对于包含 Au-DP/TS-1 和 Au-PVP/TS-1 的数据集,此活动站点模型估计的周转频率比先前报告的最高估计高 ∼20 倍(∼80 s –1 vs 1-5小号–1在 473 K) 用于贵金属的催化氧化,这表明仅在近端 Au-Ti 位点上发生的同时机制无法解释观察到的丙烯环氧化速率,并且过氧化氢的短程迁移与动力学相关。Au-DP/TS-1 和 Au-PVP/TS-1 上丙烯环氧化的反应级数、活化能和活性位点模型的一致性表明了丙烯环氧化在含有小孔内金簇的催化剂和催化剂上的共同机制仅具有更大的晶外金纳米颗粒。发现氢氧化速率与表面金原子的数量成比例变化。
更新日期:2022-08-04
中文翻译:

AU/TS-1催化剂上晶外金活性位点的丙烯环氧化动力学
为了确定负载在钛硅酸盐-1 (TS-1) 上的晶外和晶内金纳米粒子对直接丙烯环氧化的催化作用是否存在本质上的不同,在气相连续搅拌釜反应器 (CSTR) 中测量了直接丙烯环氧化的动力学。在沉积在 TS-1 上的聚乙烯吡咯烷酮涂层 (PVP) 金纳米粒子 (Au-PVP/TS-1) 上。正如TEM 和 TGA-DSC 所证实的那样,即使在通过一系列预处理原位去除配体后,制成的 PVP 涂层金纳米颗粒也太大而无法装入 TS-1 的微孔中。H2 (1.3)、O 2的活化能 (51 kJ mol –1 ) 和反应级数(0.4)、丙烯 (0.4)、环氧丙烷 (-0.6)、二氧化碳 (0) 和水 (0) 用于在 Au-PVP/TS-1 上测量的丙烯环氧化与报告的 Au/TS-1 一致通过沉积-沉淀法制备 (Au-DP/TS-1) (52 kJ mol –1 , H 2 , 1; O 2 , 0.4; C 3 H 6 , 0.4; C 3 H 6 O, -0.6; CO 2 , 0;H 2 O,0)。然而,虽然 Au-PVP/TS-1 上的氢氧化反应顺序 (H 2 , 0.8; O 2 , 0; C 3 H 6 , -0.3; C 3 H 6 O, -0.1; CO 2 , 0; H2 O, -0.2) 与在 Au-DP/TS-1 上测量的相似 (H 2 , 0.9; O 2 , 0.3; C 3 H 6 , -0.3; C 3 H 6 O, 0; CO 2 , 0 ; H 2 O, -0.1),活化能从 Au-DP/TS-1 的大约 30 kJ mol –1降低到 4–5 kJ mol –1Au-PVP/TS-1 表明限速步骤或氢氧化的活性位点发生了变化。此外,还开发了一个活性位点模型,该模型确定了晶外 Au 纳米颗粒周边相互作用范围内的 Ti 数量(即,Au-Ti 活性位点对的数量)。该模型用于估计仅近端 Au-Ti 对上的催化转换频率,假设过氧化氢不会从 Au 位点解吸和迁移到 Ti 位点,而是以先前称为“同时”机制的协同机制形成环氧丙烷。对于包含 Au-DP/TS-1 和 Au-PVP/TS-1 的数据集,此活动站点模型估计的周转频率比先前报告的最高估计高 ∼20 倍(∼80 s –1 vs 1-5小号–1在 473 K) 用于贵金属的催化氧化,这表明仅在近端 Au-Ti 位点上发生的同时机制无法解释观察到的丙烯环氧化速率,并且过氧化氢的短程迁移与动力学相关。Au-DP/TS-1 和 Au-PVP/TS-1 上丙烯环氧化的反应级数、活化能和活性位点模型的一致性表明了丙烯环氧化在含有小孔内金簇的催化剂和催化剂上的共同机制仅具有更大的晶外金纳米颗粒。发现氢氧化速率与表面金原子的数量成比例变化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号