当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Single Residue K12 Governs the Exceptional Voltage Sensitivity of Mitochondrial Voltage-Dependent Anion Channel Gating
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-04 , DOI: 10.1021/jacs.2c03316 Van A Ngo 1, 2 , María Queralt-Martín 3, 4 , Farha Khan 5 , Lucie Bergdoll 6 , Jeff Abramson 5 , Sergey M Bezrukov 3 , Tatiana K Rostovtseva 3 , David P Hoogerheide 7 , Sergei Yu Noskov 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-04 , DOI: 10.1021/jacs.2c03316 Van A Ngo 1, 2 , María Queralt-Martín 3, 4 , Farha Khan 5 , Lucie Bergdoll 6 , Jeff Abramson 5 , Sergey M Bezrukov 3 , Tatiana K Rostovtseva 3 , David P Hoogerheide 7 , Sergei Yu Noskov 1
Affiliation
|
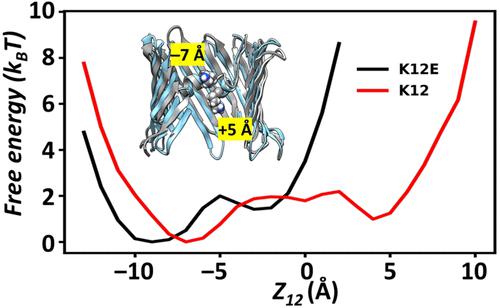
The voltage-dependent anion channel (VDAC) is a β-barrel channel of the mitochondrial outer membrane (MOM) that passively transports ions, metabolites, polypeptides, and single-stranded DNA. VDAC responds to a transmembrane potential by “gating,” i.e. transitioning to one of a variety of low-conducting states of unknown structure. The gated state results in nearly complete suppression of multivalent mitochondrial metabolite (such as ATP and ADP) transport, while enhancing calcium transport. Voltage gating is a universal property of β-barrel channels, but VDAC gating is anomalously sensitive to transmembrane potential. Here, we show that a single residue in the pore interior, K12, is responsible for most of VDAC’s voltage sensitivity. Using the analysis of over 40 μs of atomistic molecular dynamics (MD) simulations, we explore correlations between motions of charged residues inside the VDAC pore and geometric deformations of the β-barrel. Residue K12 is bistable; its motions between two widely separated positions along the pore axis enhance the fluctuations of the β-barrel and augment the likelihood of gating. Single channel electrophysiology of various K12 mutants reveals a dramatic reduction of the voltage-induced gating transitions. The crystal structure of the K12E mutant at a resolution of 2.6 Å indicates a similar architecture of the K12E mutant to the wild type; however, 60 μs of atomistic MD simulations using the K12E mutant show restricted motion of residue 12, due to enhanced connectivity with neighboring residues, and diminished amplitude of barrel motions. We conclude that β-barrel fluctuations, governed particularly by residue K12, drive VDAC gating transitions.
中文翻译:

单个残基 K12 控制线粒体电压依赖性阴离子通道门控的异常电压敏感性
电压依赖性阴离子通道 (VDAC) 是线粒体外膜 (MOM) 的 β 桶通道,它被动地转运离子、代谢物、多肽和单链 DNA。VDAC 通过“门控”响应跨膜电位,即转变为未知结构的多种低导电状态之一。门控状态导致几乎完全抑制多价线粒体代谢物(如 ATP 和 ADP)转运,同时增强钙转运。电压门控是 β 桶通道的普遍特性,但 VDAC 门控对跨膜电位异常敏感。在这里,我们展示了孔隙内部的单个残留物 K12 是 VDAC 电压敏感性的主要原因。使用超过 40 μs 的原子分子动力学 (MD) 模拟分析,我们探讨了 VDAC 孔内带电残基的运动与 β-桶的几何变形之间的相关性。残基 K12 是双稳态的;它沿孔轴在两个相距很远的位置之间的运动增强了 β 桶的波动并增加了门控的可能性。各种 K12 突变体的单通道电生理学显示电压诱导的门控转换显着减少。分辨率为 2.6 Å 的 K12E 突变体的晶体结构表明 K12E 突变体的结构与野生型相似。然而,使用 K12E 突变体的 60 μs 原子 MD 模拟显示残基 12 的运动受限,这是由于与相邻残基的连接性增强,以及桶运动幅度减小。我们得出结论,尤其受残基 K12 控制的 β-桶波动,
更新日期:2022-08-04
中文翻译:

单个残基 K12 控制线粒体电压依赖性阴离子通道门控的异常电压敏感性
电压依赖性阴离子通道 (VDAC) 是线粒体外膜 (MOM) 的 β 桶通道,它被动地转运离子、代谢物、多肽和单链 DNA。VDAC 通过“门控”响应跨膜电位,即转变为未知结构的多种低导电状态之一。门控状态导致几乎完全抑制多价线粒体代谢物(如 ATP 和 ADP)转运,同时增强钙转运。电压门控是 β 桶通道的普遍特性,但 VDAC 门控对跨膜电位异常敏感。在这里,我们展示了孔隙内部的单个残留物 K12 是 VDAC 电压敏感性的主要原因。使用超过 40 μs 的原子分子动力学 (MD) 模拟分析,我们探讨了 VDAC 孔内带电残基的运动与 β-桶的几何变形之间的相关性。残基 K12 是双稳态的;它沿孔轴在两个相距很远的位置之间的运动增强了 β 桶的波动并增加了门控的可能性。各种 K12 突变体的单通道电生理学显示电压诱导的门控转换显着减少。分辨率为 2.6 Å 的 K12E 突变体的晶体结构表明 K12E 突变体的结构与野生型相似。然而,使用 K12E 突变体的 60 μs 原子 MD 模拟显示残基 12 的运动受限,这是由于与相邻残基的连接性增强,以及桶运动幅度减小。我们得出结论,尤其受残基 K12 控制的 β-桶波动,











































 京公网安备 11010802027423号
京公网安备 11010802027423号