当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gram-Scale Asymmetric Synthesis of Fluorinated Amino Acids Using a Chiral Nickel(II) Complex
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.joc.2c00522 Thomas Hohmann 1 , Michael Dyrks 1 , Suvrat Chowdhary 1 , Manuela Weber 2 , Duy Nguyen 1 , Johann Moschner 1 , Beate Koksch 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.joc.2c00522 Thomas Hohmann 1 , Michael Dyrks 1 , Suvrat Chowdhary 1 , Manuela Weber 2 , Duy Nguyen 1 , Johann Moschner 1 , Beate Koksch 1
Affiliation
|
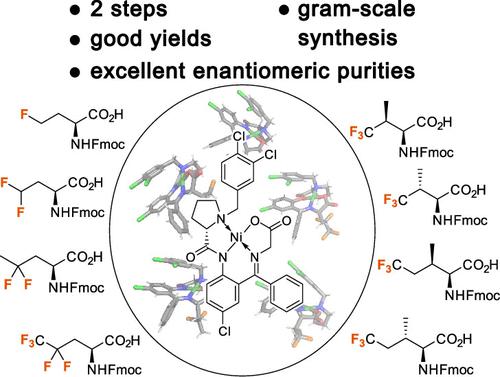
Fluorinated amino acids play an important role in the field of peptide and protein engineering. Although numerous syntheses have been published in recent decades, strategies that allow routine access to fluorinated amino acids on a gram-scale have been poorly described. Furthermore, the described pathways that gain fluorinated amino acids are based on different synthetic strategies, making a uniform approach that uses similar starting materials highly beneficial. Chiral Ni(II) complexes were introduced as powerful tools in the synthesis of noncanonical amino acids. In this work, we present a strategy for the synthesis of a diverse range of fluorinated amino acids based on the corresponding Ni(II) complex from which the products can be obtained in enantiopure form (99% ee) on a gram-scale. In addition, we describe an optimized procedure for the synthesis of alkyl iodide building blocks that are required for the alkylation reactions with the corresponding Ni(II) complex. Finally, we characterized the synthesized fluorinated amino acids with regard to their hydrophobicity and α-helix propensity.
中文翻译:

使用手性镍 (II) 配合物的克级不对称合成氟化氨基酸
氟化氨基酸在肽和蛋白质工程领域发挥着重要作用。尽管近几十年来已经发表了许多合成方法,但很少描述允许常规获取克级氟化氨基酸的策略。此外,所描述的获得氟化氨基酸的途径基于不同的合成策略,使得使用相似起始材料的统一方法非常有益。手性 Ni(II) 配合物作为合成非常规氨基酸的有力工具被引入。在这项工作中,我们提出了一种基于相应的 Ni(II) 配合物合成多种氟化氨基酸的策略,从中可以得到克级对映体纯形式 (99% ee) 的产物。此外,我们描述了合成烷基碘结构单元的优化程序,这些结构单元是与相应的 Ni (II) 配合物进行烷基化反应所需的。最后,我们对合成的氟化氨基酸的疏水性和α-螺旋倾向进行了表征。
更新日期:2022-08-04
中文翻译:

使用手性镍 (II) 配合物的克级不对称合成氟化氨基酸
氟化氨基酸在肽和蛋白质工程领域发挥着重要作用。尽管近几十年来已经发表了许多合成方法,但很少描述允许常规获取克级氟化氨基酸的策略。此外,所描述的获得氟化氨基酸的途径基于不同的合成策略,使得使用相似起始材料的统一方法非常有益。手性 Ni(II) 配合物作为合成非常规氨基酸的有力工具被引入。在这项工作中,我们提出了一种基于相应的 Ni(II) 配合物合成多种氟化氨基酸的策略,从中可以得到克级对映体纯形式 (99% ee) 的产物。此外,我们描述了合成烷基碘结构单元的优化程序,这些结构单元是与相应的 Ni (II) 配合物进行烷基化反应所需的。最后,我们对合成的氟化氨基酸的疏水性和α-螺旋倾向进行了表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号