当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Cyclization of Alkynyl Enaminones: Controllable Synthesis of Indeno[1,2-c]pyrroles or Indanones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.joc.2c01373 Yulei Zhao 1 , Yuhang Fan 1 , Xiaohan Meng 1 , Xin Kang 1 , Zhongyin Ji 1 , Shina Yan 1 , Laijin Tian 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.joc.2c01373 Yulei Zhao 1 , Yuhang Fan 1 , Xiaohan Meng 1 , Xin Kang 1 , Zhongyin Ji 1 , Shina Yan 1 , Laijin Tian 1
Affiliation
|
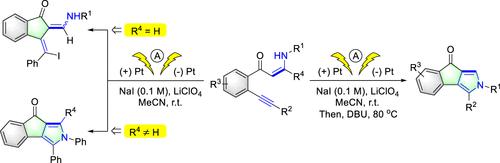
We report an electrochemical intramolecular [3 + 2] cyclization of alkynyl enaminones in a user-friendly undivided cell under constant current conditions without an oxidant and catalyst, and indeno[1,2-c]pyrrole derivatives could be obtained in good to excellent yields. Notably, preliminary substituent-controlled selective transformation is also achieved under electrocatalysis alone, and indeno[1,2-c]pyrrole (R4 ≠ H) or indanone derivatives (R4 = H) could be prepared directly under electrocatalysis without adding a base and heating process.
中文翻译:

炔基烯胺酮的电化学环化:茚并[1,2-c]吡咯或茚满酮的可控合成
我们报告了在没有氧化剂和催化剂的恒定电流条件下,在用户友好的未分裂电池中,炔基烯胺酮的电化学分子内 [3 + 2] 环化,并且可以以良好至优异的产率获得茚并[1,2- c ]吡咯衍生物. 值得注意的是,仅在电催化下也可以实现初步的取代基控制的选择性转化,并且可以在不添加碱的情况下直接在电催化下制备茚并[1,2- c ]吡咯(R 4 ≠ H)或茚满酮衍生物(R 4 = H)和加热过程。
更新日期:2022-08-04
中文翻译:

炔基烯胺酮的电化学环化:茚并[1,2-c]吡咯或茚满酮的可控合成
我们报告了在没有氧化剂和催化剂的恒定电流条件下,在用户友好的未分裂电池中,炔基烯胺酮的电化学分子内 [3 + 2] 环化,并且可以以良好至优异的产率获得茚并[1,2- c ]吡咯衍生物. 值得注意的是,仅在电催化下也可以实现初步的取代基控制的选择性转化,并且可以在不添加碱的情况下直接在电催化下制备茚并[1,2- c ]吡咯(R 4 ≠ H)或茚满酮衍生物(R 4 = H)和加热过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号