当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Redox-Neutral Generation of Iminyl Radicals by N-Heterocyclic Carbene Catalysis: Rapid Access to Phenanthridines from Vinyl Azides
Organic Letters ( IF 5.2 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.orglett.2c02118 Lixia Liu 1 , Qijing Zhang 1 , Chengming Wang 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.orglett.2c02118 Lixia Liu 1 , Qijing Zhang 1 , Chengming Wang 1
Affiliation
|
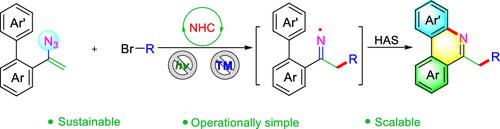
An N-heterocyclic carbene-catalyzed oxidant-, metal- and light-free iminyl radical generation pathway stemming from the reaction of vinyl azide and α-bromo ester is uncovered. This newly developed methodology is successfully applied to the redox-neutral construction of a number of diversified phenanthridine derivatives with nice functional group compatibility. Insights from the mechanism study reveal that this NHC-catalyzed transformation potentially proceeds through an alkyl radical addition-initiated HAS process, with the iminyl radical as an active intermediate.
中文翻译:

N-杂环卡宾催化氧化还原中性生成亚胺基:从叠氮化乙烯基中快速获得菲啶
揭示了源自乙烯基叠氮化物和 α-溴酯反应的N-杂环卡宾催化的氧化剂、金属和无光亚胺基自由基生成途径。这种新开发的方法成功地应用于具有良好官能团相容性的多种菲啶衍生物的氧化还原中性构建。机理研究表明,这种 NHC 催化的转化可能通过烷基自由基加成引发的 HAS 过程进行,亚胺自由基作为活性中间体。
更新日期:2022-08-04
中文翻译:

N-杂环卡宾催化氧化还原中性生成亚胺基:从叠氮化乙烯基中快速获得菲啶
揭示了源自乙烯基叠氮化物和 α-溴酯反应的N-杂环卡宾催化的氧化剂、金属和无光亚胺基自由基生成途径。这种新开发的方法成功地应用于具有良好官能团相容性的多种菲啶衍生物的氧化还原中性构建。机理研究表明,这种 NHC 催化的转化可能通过烷基自由基加成引发的 HAS 过程进行,亚胺自由基作为活性中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号