当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling Intracellular Enzymatic Self-Assembly of Peptide by Host–Guest Complexation for Programming Cancer Cell Death
Nano Letters ( IF 9.6 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.nanolett.2c02612 Xuejiao Yang 1 , Bihan Wu 1, 2 , Jiong Zhou 3 , Honglei Lu 1 , Hongyue Zhang 1 , Feihe Huang 3, 4, 5 , Huaimin Wang 1
Nano Letters ( IF 9.6 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.nanolett.2c02612 Xuejiao Yang 1 , Bihan Wu 1, 2 , Jiong Zhou 3 , Honglei Lu 1 , Hongyue Zhang 1 , Feihe Huang 3, 4, 5 , Huaimin Wang 1
Affiliation
|
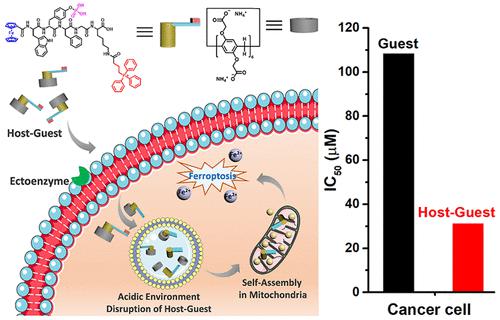
Controlling the enzymatic reaction of macromolecules in living systems plays an essential role in determining the biological functions, which remains challenging in the synthetic system. This work shows that host–guest complexation could be an efficient strategy to tune the enzymatic self-assembly of the peptide. The formed host–guest complexation prevents the enzymatic kinetics of peptide assemblies on the cell surface and promotes cellular uptake of assemblies. For uptake inside cells, the host–guest complex undergoes dissociation in the acidic lysosome, and the released peptide further self-assembles inside the mitochondria. Accumulating assemblies at mitochondria induce the ferroptosis of cancer cells, resulting in cancer cell death in vitro and the tumor-bearing mice model. As the first example of using host–guest complexation to modulate the kinetics of enzymatic self-assembly, this work provides a general method to control enzymatic self-assembly in living cells for selective programming cancer cell death.
中文翻译:

通过宿主-客体络合控制肽的细胞内酶促自组装以编程癌细胞死亡
控制生命系统中大分子的酶促反应在确定生物功能方面起着至关重要的作用,这在合成系统中仍然具有挑战性。这项工作表明,主客体复合可能是调节肽酶促自组装的有效策略。形成的主客体复合物阻止了细胞表面肽组装体的酶动力学,并促进了细胞对组装体的吸收。对于细胞内的摄取,主客体复合物在酸性溶酶体中解离,释放的肽在线粒体内进一步自组装。在线粒体中积累的组装体诱导癌细胞的铁死亡,导致癌细胞在体外死亡和荷瘤小鼠模型。作为使用主客体络合调节酶促自组装动力学的第一个例子,这项工作提供了一种控制活细胞中酶促自组装以选择性编程癌细胞死亡的通用方法。
更新日期:2022-08-04
中文翻译:

通过宿主-客体络合控制肽的细胞内酶促自组装以编程癌细胞死亡
控制生命系统中大分子的酶促反应在确定生物功能方面起着至关重要的作用,这在合成系统中仍然具有挑战性。这项工作表明,主客体复合可能是调节肽酶促自组装的有效策略。形成的主客体复合物阻止了细胞表面肽组装体的酶动力学,并促进了细胞对组装体的吸收。对于细胞内的摄取,主客体复合物在酸性溶酶体中解离,释放的肽在线粒体内进一步自组装。在线粒体中积累的组装体诱导癌细胞的铁死亡,导致癌细胞在体外死亡和荷瘤小鼠模型。作为使用主客体络合调节酶促自组装动力学的第一个例子,这项工作提供了一种控制活细胞中酶促自组装以选择性编程癌细胞死亡的通用方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号