当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-Deoxyglycosides Bearing Free Hydroxyl Substituents on the Glycosyl Donor
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.joc.2c01003 Kevin M Hoang 1 , Xiaoying Zheng 1 , Seth B Herzon 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.joc.2c01003 Kevin M Hoang 1 , Xiaoying Zheng 1 , Seth B Herzon 1, 2
Affiliation
|
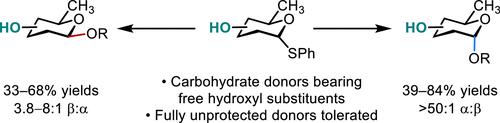
Recent efforts in the field of carbohydrate chemistry have focused on the site- and stereocontrolled synthesis of O-glycosides derived from acceptors bearing multiple hydroxyl substituents. By comparison, there are few examples of the site-selective synthesis of O-glycosides bearing free hydroxyl substituents on the donor reagent. Here, we report the application of an umpolung glycosylation strategy to the synthesis of O-glycosides derived from donors bearing free hydroxyl substituents. The reaction proceeds via prior deprotonation of one or more free hydroxyl groups on a thiophenylglycoside donor, reductive lithiation to generate an anomeric anion intermediate, and addition of this anion to an alkyl 2-(2-methyltetrahydropyranyl) peroxide. By this approach, α-linked glycosides were obtained in 39–84% yields and with >50:1 α/β selectivities. In many instances, β-linked products could be obtained by thermal equilibration of the anomeric anion intermediate (selectivities = 3.8–8:1 β/α; yields = 33–68%). The strategy is applicable to polyhydroxyl donors bearing up to three free hydroxyl groups, N-acylated carbohydrates, and the single-flask syntheses of oligosaccharides.
中文翻译:

糖基供体上带有游离羟基取代基的2-脱氧糖苷的合成
最近在碳水化合物化学领域的努力集中在从带有多个羟基取代基的受体衍生的O-糖苷的位点和立体控制合成上。相比之下,在供体试剂上选择性合成带有游离羟基取代基的O-糖苷的例子很少。在这里,我们报告了 umpolung 糖基化策略在O合成中的应用-衍生自带有游离羟基取代基的供体的糖苷。该反应通过预先对苯硫基糖苷供体上的一个或多个游离羟基进行去质子化、还原锂化以产生异头阴离子中间体并将该阴离子添加到烷基2-(2-甲基四氢吡喃)过氧化物中而进行。通过这种方法,以 39-84% 的产率获得了 α-连接的糖苷,并且具有 >50:1 的 α/β 选择性。在许多情况下,β-连接的产物可以通过异头阴离子中间体的热平衡获得(选择性 = 3.8–8:1 β/α;产率 = 33–68%)。该策略适用于带有最多三个游离羟基的多羟基供体、N-酰化碳水化合物和寡糖的单瓶合成。
更新日期:2022-08-03
中文翻译:

糖基供体上带有游离羟基取代基的2-脱氧糖苷的合成
最近在碳水化合物化学领域的努力集中在从带有多个羟基取代基的受体衍生的O-糖苷的位点和立体控制合成上。相比之下,在供体试剂上选择性合成带有游离羟基取代基的O-糖苷的例子很少。在这里,我们报告了 umpolung 糖基化策略在O合成中的应用-衍生自带有游离羟基取代基的供体的糖苷。该反应通过预先对苯硫基糖苷供体上的一个或多个游离羟基进行去质子化、还原锂化以产生异头阴离子中间体并将该阴离子添加到烷基2-(2-甲基四氢吡喃)过氧化物中而进行。通过这种方法,以 39-84% 的产率获得了 α-连接的糖苷,并且具有 >50:1 的 α/β 选择性。在许多情况下,β-连接的产物可以通过异头阴离子中间体的热平衡获得(选择性 = 3.8–8:1 β/α;产率 = 33–68%)。该策略适用于带有最多三个游离羟基的多羟基供体、N-酰化碳水化合物和寡糖的单瓶合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号