Journal of Controlled Release ( IF 10.5 ) Pub Date : 2022-08-03 , DOI: 10.1016/j.jconrel.2022.07.041 Annabelle Biscans 1 , Socheata Ly 1 , Nicholas McHugh 1 , David A Cooper 1 , Anastasia Khvorova 1
|
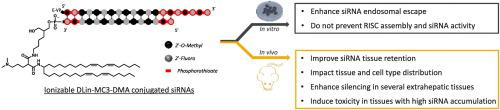
Lipid conjugation supports delivery of small interfering RNAs (siRNAs) to extrahepatic tissues, expanding the therapeutic potential of siRNAs beyond liver indications. However, siRNA silencing efficacy in extrahepatic tissues remains inferior to that routinely achieved in liver, partially due to the low rate of endosomal escape following siRNA internalization. Improving siRNA endosomal release into cytoplasm is crucial to improving efficacy of lipid-conjugated siRNAs. Given the ability of ionizable lipids to enhance endosomal escape in a context of lipid nanoparticles (LNP), here, we provide the first report on the effect of an ionizable lipid conjugate on siRNA endosomal escape, tissue distribution, efficacy, and toxicity in vivo. After developing a synthetic route to covalently attach the ionizable lipid, DLin-MC3-DMA, to siRNAs, we demonstrate that DLin-MC3-DMA enhances endosomal escape in cell culture without compromising siRNA efficacy. In mice, DLin-MC3-DMA conjugated siRNAs exhibit a similar overall tissue distribution profile to the similarly hydrophobic cholesterol-conjugated siRNA. However, only DLin-MC3-DMA conjugated siRNAs accumulated in vascular compartments, suggesting an effect of conjugate structure on intratissue distribution. Interestingly, we observed non-specific modulation of gene expression in tissues with high accumulation of DLin-MC3-DMA siRNAs (>20 pmol/mg of tissue) while limited non-specific gene modulation has been observed in tissues with lower siRNA accumulation. These findings suggest modulating the nature of the conjugate is a promising strategy to alter siRNA intratissue and intracellular trafficking. Fine-tuning the nature of the conjugate to optimize endosomal escape while minimizing toxicity will be critical for the progression of therapeutic siRNA applications beyond the liver.
中文翻译:

工程化可电离脂质 siRNA 缀合物增强内体逃逸,但会引起体内毒性
脂质缀合支持将小干扰 RNA (siRNA) 递送至肝外组织,从而将 siRNA 的治疗潜力扩展到肝脏适应症之外。然而,肝外组织中的 siRNA 沉默效果仍然不如肝脏中常规达到的效果,部分原因是 siRNA 内化后内体逃逸率较低。改善 siRNA 内体释放到细胞质中对于提高脂质缀合 siRNA 的功效至关重要。鉴于可电离脂质在脂质纳米颗粒 (LNP) 的背景下增强内体逃逸的能力,在此,我们提供了第一份关于可电离脂质缀合物对 siRNA 内体逃逸、组织分布、功效和体内毒性影响的报告。在开发了一种将可电离脂质 DLin-MC3-DMA 共价连接到 siRNA 上的合成途径后,我们证明 DLin-MC3-DMA 可以增强细胞培养物中的内体逃逸,而不会影响 siRNA 的功效。在小鼠中,DLin-MC3-DMA 缀合的 siRNA 表现出与疏水性胆固醇缀合 siRNA 相似的整体组织分布特征。然而,只有DLin-MC3-DMA缀合的siRNA在血管区室中积累,这表明缀合物结构对组织内分布的影响。有趣的是,我们在 DLin-MC3-DMA siRNA 高积累(>20 pmol/mg 组织)的组织中观察到基因表达的非特异性调节,而在 siRNA 积累较低的组织中观察到有限的非特异性基因调节。这些发现表明,调节缀合物的性质是改变 siRNA 组织内和细胞内运输的一种有前景的策略。微调缀合物的性质以优化内体逃逸,同时最大限度地降低毒性对于 siRNA 在肝脏以外的应用的进展至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号