当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into Criegee Intermediate–Hydroperoxyl Radical Chemistry
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-03 , DOI: 10.1021/jacs.2c05346 Bai Li 1 , Manoj Kumar 2 , Chuan Zhou 1 , Lei Li 1 , Joseph S Francisco 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-03 , DOI: 10.1021/jacs.2c05346 Bai Li 1 , Manoj Kumar 2 , Chuan Zhou 1 , Lei Li 1 , Joseph S Francisco 2
Affiliation
|
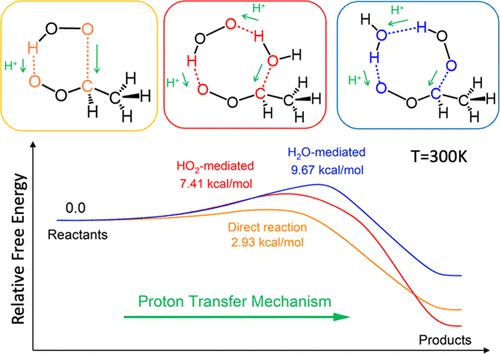
The reaction between a Criegee intermediate and the hydroperoxyl radical (HO2) is believed to play a role in the formation of new particles in the troposphere. Although the reaction has been previously studied in the gas phase, there are several knowledge gaps that still need to be filled. We simulated the reaction of anti-CH3CHOO with HO2 and HO2–H2O radical complexes in the gas phase at 0 K, which exhibited a low-barrier profile for water-containing systems and a barrierless profile for water-free systems. Moreover, the reaction was found to follow a proton-transfer mechanism, which challenges previous assumptions that the gas-phase reaction involves a hydrogen atom transfer. The HO2 radical was found to mediate the Criegee hydration reaction in the gas phase. Metadynamics simulations further confirmed that the expected radical adduct formation between anti-CH3CHOO and the HO2 radical, as well as the HO2- and H2O-mediated reactions in the gas phase, followed a concerted mechanism. By combining constrained ab initio molecular dynamics simulations with thermodynamic integration, we quantitively evaluated the free-energy barriers at high temperatures. The barriers obtained for all three Criegee–HO2 reaction systems were found to be temperature-dependent. We also compared the free-energy barriers of water-free and water-containing systems; the results revealed that water could hinder the reaction between the Criegee and HO2 radical. These results suggest that HO2 radicals may be involved in the formation of tropospheric radical adducts, and water molecules may also play important roles in the reactions of Criegee intermediates.
中文翻译:

Criegee 中间体-羟基自由基化学的机理研究
Criegee 中间体与氢过氧自由基 (HO 2 ) 之间的反应被认为在对流层中新粒子的形成中起作用。尽管之前已经在气相中研究过该反应,但仍有一些知识空白需要填补。我们模拟了抗-CH 3 CHOO 与HO 2和HO 2 –H 2的反应在 0 K 的气相中的 O 自由基配合物,在含水系统中表现出低阻隔分布,而在无水系统中表现出无阻隔分布。此外,发现该反应遵循质子转移机制,这挑战了之前气相反应涉及氢原子转移的假设。发现HO 2自由基介导气相中的Criegee水合反应。元动力学模拟进一步证实了预期的抗-CH 3 CHOO 和HO 2自由基之间的自由基加合物形成,以及气相中HO 2 - 和H 2 O 介导的反应遵循一致的机制。通过结合约束通过热力学积分的从头算分子动力学模拟,我们定量评估了高温下的自由能垒。发现所有三个 Criegee-HO 2反应系统获得的势垒都与温度有关。我们还比较了无水和含水系统的自由能势垒;结果表明,水会阻碍 Criegee 和 HO 2自由基之间的反应。这些结果表明,HO 2自由基可能参与了对流层自由基加合物的形成,水分子也可能在 Criegee 中间体的反应中发挥重要作用。
更新日期:2022-08-03
中文翻译:

Criegee 中间体-羟基自由基化学的机理研究
Criegee 中间体与氢过氧自由基 (HO 2 ) 之间的反应被认为在对流层中新粒子的形成中起作用。尽管之前已经在气相中研究过该反应,但仍有一些知识空白需要填补。我们模拟了抗-CH 3 CHOO 与HO 2和HO 2 –H 2的反应在 0 K 的气相中的 O 自由基配合物,在含水系统中表现出低阻隔分布,而在无水系统中表现出无阻隔分布。此外,发现该反应遵循质子转移机制,这挑战了之前气相反应涉及氢原子转移的假设。发现HO 2自由基介导气相中的Criegee水合反应。元动力学模拟进一步证实了预期的抗-CH 3 CHOO 和HO 2自由基之间的自由基加合物形成,以及气相中HO 2 - 和H 2 O 介导的反应遵循一致的机制。通过结合约束通过热力学积分的从头算分子动力学模拟,我们定量评估了高温下的自由能垒。发现所有三个 Criegee-HO 2反应系统获得的势垒都与温度有关。我们还比较了无水和含水系统的自由能势垒;结果表明,水会阻碍 Criegee 和 HO 2自由基之间的反应。这些结果表明,HO 2自由基可能参与了对流层自由基加合物的形成,水分子也可能在 Criegee 中间体的反应中发挥重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号