当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diversification of (E,E)-1,6-Dioxo-2,4-Dienes for the Synthesis of (+)-Aspicillin, Isolaurepan, and β-Parinaric Acid
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.joc.2c01280 Dattatraya H Dethe 1 , Aparna Srivastava 1 , Appasaheb K Nirpal 1 , Nagabhushana C Beeralingappa 1 , Vimlesh Kumar 1 , Arsheed A Bhat 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.joc.2c01280 Dattatraya H Dethe 1 , Aparna Srivastava 1 , Appasaheb K Nirpal 1 , Nagabhushana C Beeralingappa 1 , Vimlesh Kumar 1 , Arsheed A Bhat 1
Affiliation
|
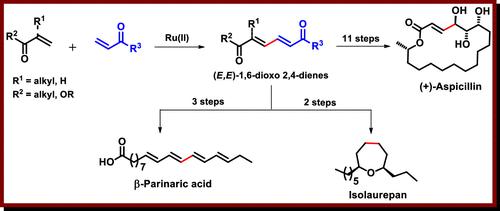
A divergent formal synthesis of polyhydroxylated macrocyclic lactone (+)-aspicillin and polyene bioactive natural product β-parinaric acid and the total synthesis of non-terpenoid metabolite isolaurepan have been achieved using a ruthenium-catalyzed stereo- and chemoselective oxidative coupling reaction of easily accessible vinyl ketones and acrylates. The crucial transformation involves the efficient synthesis and functionalization of stereodefined (E,E)-1,6-dioxo-2,4-dienes using simple reaction protocols, which enabled straightforward access to a diverse range of bioactive natural products.
中文翻译:

(E,E)-1,6-Dioxo-2,4-Dienes 的多样化用于 (+)-Aspicillin、Isolaurepan 和 β-Parinaric 酸的合成
使用容易获得的钌催化的立体和化学选择性氧化偶联反应,实现了多羟基化大环内酯 (+)-阿司匹林和多烯生物活性天然产物 β-parinaric 酸的不同形式合成和非萜类代谢物异拉脲的全合成。乙烯基酮和丙烯酸酯。关键的转化涉及使用简单的反应方案对立体定义的 ( E,E )-1,6-dioxo-2,4-二烯进行有效合成和功能化,从而可以直接获得各种生物活性天然产物。
更新日期:2022-08-03
中文翻译:

(E,E)-1,6-Dioxo-2,4-Dienes 的多样化用于 (+)-Aspicillin、Isolaurepan 和 β-Parinaric 酸的合成
使用容易获得的钌催化的立体和化学选择性氧化偶联反应,实现了多羟基化大环内酯 (+)-阿司匹林和多烯生物活性天然产物 β-parinaric 酸的不同形式合成和非萜类代谢物异拉脲的全合成。乙烯基酮和丙烯酸酯。关键的转化涉及使用简单的反应方案对立体定义的 ( E,E )-1,6-dioxo-2,4-二烯进行有效合成和功能化,从而可以直接获得各种生物活性天然产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号