当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni/Photoredox-Catalyzed C(sp2)–C(sp3) Cross-Coupling of Alkyl Pinacolboronates and (Hetero)Aryl Bromides
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.orglett.2c01942 Anthony N Cauley 1, 2 , Antonio Ramirez 1 , Chandan L Barhate 2 , Andrew F Donnell 2 , Purnima Khandelwal 2 , Melda Sezen-Edmonds 1 , Trevor C Sherwood 2 , Jack L Sloane 2 , Cullen L Cavallaro 2 , Eric M Simmons 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.orglett.2c01942 Anthony N Cauley 1, 2 , Antonio Ramirez 1 , Chandan L Barhate 2 , Andrew F Donnell 2 , Purnima Khandelwal 2 , Melda Sezen-Edmonds 1 , Trevor C Sherwood 2 , Jack L Sloane 2 , Cullen L Cavallaro 2 , Eric M Simmons 1
Affiliation
|
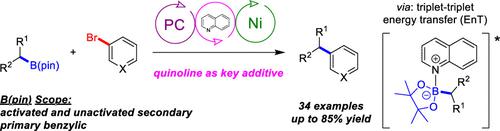
Utilizing quinoline as a mild, catalytic additive, broadly applicable conditions for the Ni/photoredox-catalyzed C(sp2)–C(sp3) cross-coupling of (hetero)aryl bromides and alkyl pinacolboronate esters were developed, which can be applied to both batch and flow reactions. In addition to primary benzylic nucleophiles, both stabilized and nonstabilized secondary alkyl boronic esters are effective coupling partners. Density functional theory calculations suggest that alkyl radical generation occurs from an alkyl-B(pin)-quinoline complex, which may proceed via an energy transfer process.
更新日期:2022-08-03











































 京公网安备 11010802027423号
京公网安备 11010802027423号