当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accurate Description of Solvent-Exposed Salt Bridges with a Non-polarizable Force Field Incorporating Solvent Effects
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.jcim.2c00678 Han Liu 1, 2 , Haohao Fu 1, 2 , Christophe Chipot 3, 4, 5 , Xueguang Shao 1, 2 , Wensheng Cai 1, 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.jcim.2c00678 Han Liu 1, 2 , Haohao Fu 1, 2 , Christophe Chipot 3, 4, 5 , Xueguang Shao 1, 2 , Wensheng Cai 1, 2
Affiliation
|
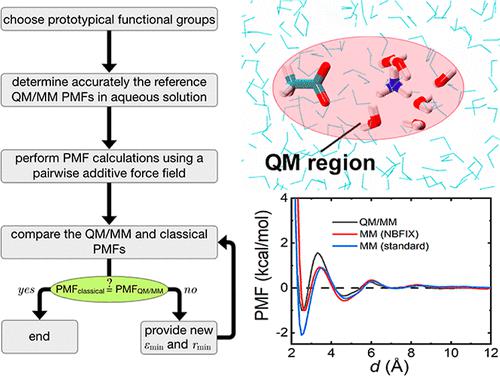
The strength of salt bridges resulting from the interaction of cations and anions is modulated by their environment. However, polarization of the solvent molecules by the charged moieties makes the accurate description of cation–anion interactions in an aqueous solution by means of a pairwise additive potential energy function and classical combination rules particularly challenging. In this contribution, aiming at improving the representation of solvent-exposed salt-bridge interactions with an all-atom non-polarizable force field, we put forth here a parametrization strategy. First, the interaction of a cation and an anion is characterized by hybrid quantum mechanical/molecular mechanics (QM/MM) potential of mean force (PMF) calculations, whereby constantly exchanging solvent molecules around the ions are treated at the quantum mechanical level. The Lennard–Jones (LJ) parameters describing the salt-bridge ion pairs are then optimized to match the reference QM/MM PMFs through the so-called nonbonded FIX, or NBFIX, feature of the CHARMM force field. We apply the new set of parameters, coined CHARMM36m-SBFIX, to the calculation of association constants for the ammonium–acetate and guanidinium–acetate complexes, the osmotic pressures for glycine zwitterions, guanidinium, and acetate ions, and to the simulation of both folded and intrinsically disordered proteins. Our findings indicate that CHARMM36m-SBFIX improves the description of solvent-exposed salt-bridge interactions, both structurally and thermodynamically. However, application of this force field to the standard binding free-energy calculation of a protein–ligand complex featuring solvent-excluded salt-bridge interactions leads to a poor reproduction of the experimental value, suggesting that the parameters optimized in an aqueous solution cannot be readily transferred to describe solvent-excluded salt-bridge interactions. Put together, owing to their sensitivity to the environment, modeling salt-bridge interactions by means of a single, universal set of LJ parameters remains a daunting theoretical challenge.
中文翻译:

包含溶剂效应的非极化力场的溶剂暴露盐桥的准确描述
由阳离子和阴离子相互作用产生的盐桥的强度受其环境的调节。然而,带电部分对溶剂分子的极化使得通过成对加性势能函数和经典组合规则准确描述水溶液中的阳离子 - 阴离子相互作用特别具有挑战性。在这项贡献中,为了改善溶剂暴露的盐桥相互作用与全原子不可极化力场的表示,我们在这里提出了一种参数化策略。首先,阳离子和阴离子的相互作用以混合量子力学/分子力学 (QM/MM) 平均力势 (PMF) 计算为特征,由此在量子力学水平上处理离子周围不断交换的溶剂分子。然后优化描述盐桥离子对的 Lennard-Jones (LJ) 参数,以通过 CHARMM 力场的所谓非键 FIX 或 NBFIX 特征匹配参考 QM/MM PMF。我们将新的参数集(称为 CHARMM36m-SBFIX)应用于计算乙酸铵和乙酸胍络合物的缔合常数、甘氨酸两性离子、胍和乙酸根离子的渗透压,并模拟折叠的和本质上无序的蛋白质。我们的研究结果表明,CHARMM36m-SBFIX 在结构和热力学上改进了对溶剂暴露的盐桥相互作用的描述。然而,将该力场应用于以溶剂排除盐桥相互作用为特征的蛋白质-配体复合物的标准结合自由能计算导致实验值的重现性差,这表明在水溶液中优化的参数不容易转移描述溶剂排除的盐桥相互作用。总而言之,由于它们对环境的敏感性,通过单一、通用的 LJ 参数集对盐桥相互作用进行建模仍然是一项艰巨的理论挑战。
更新日期:2022-08-03
中文翻译:

包含溶剂效应的非极化力场的溶剂暴露盐桥的准确描述
由阳离子和阴离子相互作用产生的盐桥的强度受其环境的调节。然而,带电部分对溶剂分子的极化使得通过成对加性势能函数和经典组合规则准确描述水溶液中的阳离子 - 阴离子相互作用特别具有挑战性。在这项贡献中,为了改善溶剂暴露的盐桥相互作用与全原子不可极化力场的表示,我们在这里提出了一种参数化策略。首先,阳离子和阴离子的相互作用以混合量子力学/分子力学 (QM/MM) 平均力势 (PMF) 计算为特征,由此在量子力学水平上处理离子周围不断交换的溶剂分子。然后优化描述盐桥离子对的 Lennard-Jones (LJ) 参数,以通过 CHARMM 力场的所谓非键 FIX 或 NBFIX 特征匹配参考 QM/MM PMF。我们将新的参数集(称为 CHARMM36m-SBFIX)应用于计算乙酸铵和乙酸胍络合物的缔合常数、甘氨酸两性离子、胍和乙酸根离子的渗透压,并模拟折叠的和本质上无序的蛋白质。我们的研究结果表明,CHARMM36m-SBFIX 在结构和热力学上改进了对溶剂暴露的盐桥相互作用的描述。然而,将该力场应用于以溶剂排除盐桥相互作用为特征的蛋白质-配体复合物的标准结合自由能计算导致实验值的重现性差,这表明在水溶液中优化的参数不容易转移描述溶剂排除的盐桥相互作用。总而言之,由于它们对环境的敏感性,通过单一、通用的 LJ 参数集对盐桥相互作用进行建模仍然是一项艰巨的理论挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号