Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, bio-evaluation, and in silico studies of some N-substituted 6-(chloro/nitro)-1H-benzimidazole derivatives as antimicrobial and anticancer agents
RSC Advances ( IF 3.9 ) Pub Date : 2022-08-03 , DOI: 10.1039/d2ra03491c Em Canh Pham 1 , Tuong Vi Thi Le 2 , Tuyen Ngoc Truong 3
RSC Advances ( IF 3.9 ) Pub Date : 2022-08-03 , DOI: 10.1039/d2ra03491c Em Canh Pham 1 , Tuong Vi Thi Le 2 , Tuyen Ngoc Truong 3
Affiliation
|
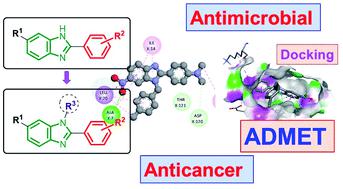
A new series of 6-substituted 1H-benzimidazole derivatives were synthesized by reacting various substituted aromatic aldehydes with 4-nitro-o-phenylenediamine and 4-chloro-o-phenylenediamine through condensation using sodium metabisulfite as the oxidative reagent. The N-substituted 6-(chloro/nitro)-1H-benzimidazole derivatives were prepared from the 6-substituted 1H-benzimidazole derivatives and substituted halides using potassium carbonate by conventional methods as well as by exposure to microwave irradiation. Seventy-six 1H-benzimidazole derivatives have been synthesized in moderate to excellent yields with the microwave-assisted method (40 to 99%). Compounds 1d, 2d, 3s, 4b, and 4k showed potent antibacterial activity against Escherichia coli, Streptococcus faecalis, MSSA (methicillin-susceptible strains of Staphylococcus aureus), and MRSA (methicillin-resistant strains of Staphylococcus aureus) with MIC (the minimum inhibitory concentration) ranging between 2 and 16 μg mL−1 as compared to ciprofloxacin (MIC = 8–16 μg mL−1), in particular compound 4k exhibits potent fungal activity against Candida albicans and Aspergillus niger with MIC ranging between 8 and 16 μg mL−1 compared with the standard drug fluconazole (MIC = 4–128 μg mL−1). In addition, compounds 1d, 2d, 3s, 4b, and 4k also showed the strongest anticancer activity among the synthesized compounds against five tested cell lines with IC50 (half-maximal inhibitory concentration) ranging between 1.84 and 10.28 μg mL−1, comparable to paclitaxel (IC50 = 1.38–6.13 μM). Furthermore, the five most active compounds showed a good ADMET (absorption, distribution, metabolism, excretion, and toxicity) profile in comparison to ciprofloxacin, fluconazole, and paclitaxel as reference drugs. Molecular docking predicted that dihydrofolate reductase protein from Staphylococcus aureus is the most suitable target for both antimicrobial and anticancer activities, and vascular endothelial growth factor receptor 2 and histone deacetylase 6 are the most suitable targets for anticancer activity of these potent compounds.
中文翻译:

一些作为抗菌剂和抗癌剂的 N-取代 6-(氯/硝基)-1H-苯并咪唑衍生物的设计、合成、生物评价和计算机研究
以焦亚硫酸钠为氧化试剂,将各种取代芳香醛与4-硝基邻苯二胺、4-氯邻苯二胺进行缩合反应,合成了一系列新的6-取代1H-苯并咪唑衍生物。 N-取代的6-(氯/硝基)-1H-苯并咪唑衍生物由6-取代的1H-苯并咪唑衍生物和取代的卤化物使用碳酸钾通过常规方法以及通过暴露于微波辐射来制备。采用微波辅助方法合成了 76 种 1 H -苯并咪唑衍生物,产率中等至优异(40% 至 99%)。化合物1d 、 2d 、 3s 、 4b和4k对大肠杆菌、粪链球菌、MSSA(甲氧西林敏感金黄色葡萄球菌菌株)和 MRSA(耐甲氧西林金黄色葡萄球菌菌株)表现出有效的抗菌活性,具有 MIC(最小抑制)与环丙沙星 (MIC = 8–16 μg mL -1 ) 相比,化合物 4k 在 2 至 16 μg mL -1之间,特别是化合物4k对白色念珠菌和黑曲霉表现出有效的真菌活性,MIC 范围在 8 至 16 μg mL -1 之间−1与标准药物氟康唑相比 (MIC = 4–128 μg mL −1 )。 此外,化合物1d 、 2d 、 3s 、 4b和4k还对五种测试细胞系显示出合成化合物中最强的抗癌活性,IC 50 (半数最大抑制浓度)范围在 1.84 至 10.28 μg mL -1之间,可比紫杉醇 (IC 50 = 1.38–6.13 μM)。此外,与作为参考药物的环丙沙星、氟康唑和紫杉醇相比,五种最活跃的化合物表现出良好的 ADMET(吸收、分布、代谢、排泄和毒性)特征。分子对接预测金黄色葡萄球菌的二氢叶酸还原酶蛋白是抗菌和抗癌活性的最合适靶点,血管内皮生长因子受体2和组蛋白脱乙酰酶6是这些有效化合物抗癌活性的最合适靶点。
更新日期:2022-08-03
中文翻译:

一些作为抗菌剂和抗癌剂的 N-取代 6-(氯/硝基)-1H-苯并咪唑衍生物的设计、合成、生物评价和计算机研究
以焦亚硫酸钠为氧化试剂,将各种取代芳香醛与4-硝基邻苯二胺、4-氯邻苯二胺进行缩合反应,合成了一系列新的6-取代1H-苯并咪唑衍生物。 N-取代的6-(氯/硝基)-1H-苯并咪唑衍生物由6-取代的1H-苯并咪唑衍生物和取代的卤化物使用碳酸钾通过常规方法以及通过暴露于微波辐射来制备。采用微波辅助方法合成了 76 种 1 H -苯并咪唑衍生物,产率中等至优异(40% 至 99%)。化合物1d 、 2d 、 3s 、 4b和4k对大肠杆菌、粪链球菌、MSSA(甲氧西林敏感金黄色葡萄球菌菌株)和 MRSA(耐甲氧西林金黄色葡萄球菌菌株)表现出有效的抗菌活性,具有 MIC(最小抑制)与环丙沙星 (MIC = 8–16 μg mL -1 ) 相比,化合物 4k 在 2 至 16 μg mL -1之间,特别是化合物4k对白色念珠菌和黑曲霉表现出有效的真菌活性,MIC 范围在 8 至 16 μg mL -1 之间−1与标准药物氟康唑相比 (MIC = 4–128 μg mL −1 )。 此外,化合物1d 、 2d 、 3s 、 4b和4k还对五种测试细胞系显示出合成化合物中最强的抗癌活性,IC 50 (半数最大抑制浓度)范围在 1.84 至 10.28 μg mL -1之间,可比紫杉醇 (IC 50 = 1.38–6.13 μM)。此外,与作为参考药物的环丙沙星、氟康唑和紫杉醇相比,五种最活跃的化合物表现出良好的 ADMET(吸收、分布、代谢、排泄和毒性)特征。分子对接预测金黄色葡萄球菌的二氢叶酸还原酶蛋白是抗菌和抗癌活性的最合适靶点,血管内皮生长因子受体2和组蛋白脱乙酰酶6是这些有效化合物抗癌活性的最合适靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号