当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbonylative Hydroacylation of Styrenes with Alkyl Halides by Multiphoton Tandem Photoredox Catalysis in Flow
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-08-02 , DOI: 10.1021/acscatal.2c02531 José A. Forni 1 , Vir H. Gandhi 1 , Anastasios Polyzos 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-08-02 , DOI: 10.1021/acscatal.2c02531 José A. Forni 1 , Vir H. Gandhi 1 , Anastasios Polyzos 1, 2
Affiliation
|
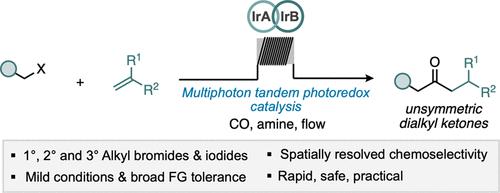
The abundance, structural diversity, and versatility of ketones give prominence to this carbonyl functional group in synthetic chemistry. The assembly of ketones via the carbonylative hydroacylation of alkenes represents a powerful modular strategy for the synthesis of unsymmetric ketone products. Here, we report the photocatalytic carbonylative hydroacylation of styrenes with unactivated alkyl halides. This protocol unifies the visible-light multiphoton catalytic cycle of [Ir(ppy)2(dtb-bpy)]+ with flow chemistry to engage energy-demanding alkyl bromides and iodides at moderate pressures of carbon monoxide. The mild and practical methodology was employed to prepare a diverse array of 44 unsymmetric dialkyl ketones from primary, secondary, and tertiary unactivated alkyl halides. We demonstrate the application of flow chemistry technology to achieve spatially resolved chemoselectivity and broad functional group tolerance for the mild generation of functionalized C(sp3)-rich ketone products.
中文翻译:

流动中多光子串联光氧化还原催化苯乙烯与烷基卤化物的羰基化加氢酰化
酮的丰度、结构多样性和多功能性使这种羰基官能团在合成化学中占有重要地位。通过烯烃的羰基化加氢酰化组装酮代表了合成不对称酮产物的强大模块化策略。在这里,我们报告了苯乙烯与未活化的卤代烷的光催化羰基氢化酰化。该协议统一了 [Ir(ppy) 2 (dtb-bpy)] +的可见光多光子催化循环借助流动化学,在中等压力的一氧化碳下使用高耗能烷基溴化物和碘化物。采用温和实用的方法,从伯、仲和叔未活化烷基卤化物中制备了 44 种不对称二烷基酮。我们展示了流动化学技术的应用,以实现空间分辨的化学选择性和广泛的官能团耐受性,以温和生成富含 C(sp 3 ) 的酮产品。
更新日期:2022-08-02
中文翻译:

流动中多光子串联光氧化还原催化苯乙烯与烷基卤化物的羰基化加氢酰化
酮的丰度、结构多样性和多功能性使这种羰基官能团在合成化学中占有重要地位。通过烯烃的羰基化加氢酰化组装酮代表了合成不对称酮产物的强大模块化策略。在这里,我们报告了苯乙烯与未活化的卤代烷的光催化羰基氢化酰化。该协议统一了 [Ir(ppy) 2 (dtb-bpy)] +的可见光多光子催化循环借助流动化学,在中等压力的一氧化碳下使用高耗能烷基溴化物和碘化物。采用温和实用的方法,从伯、仲和叔未活化烷基卤化物中制备了 44 种不对称二烷基酮。我们展示了流动化学技术的应用,以实现空间分辨的化学选择性和广泛的官能团耐受性,以温和生成富含 C(sp 3 ) 的酮产品。



























 京公网安备 11010802027423号
京公网安备 11010802027423号