当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,2-Diamines as the Amine Sources in Amidation and Rhodium-Catalyzed Asymmetric Reductive Amination Cascade Reactions
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.orglett.2c01728 Rongrong Xie 1 , Cungang Liu 1 , Renwei Lin 1 , Runchen Zhang 1 , Haizhou Huang 1 , Mingxin Chang 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.orglett.2c01728 Rongrong Xie 1 , Cungang Liu 1 , Renwei Lin 1 , Runchen Zhang 1 , Haizhou Huang 1 , Mingxin Chang 1, 2
Affiliation
|
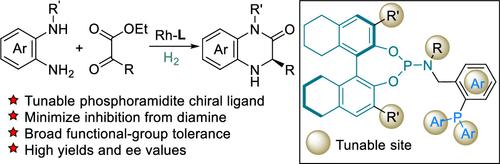
The sturdy chelation of 1,2-diamines and transition-metals would retard or even interrupt the routine catalytic cycles. In the amidation and asymmetric reductive amination (ARA) cascade reactions of diamines and ketoesters, we deployed sets of additives to ensure a smooth transformation catalyzed by the complexes of rhodium and versatile and highly modular phosphoramidite-phosphine ligands. The tunability of the ligands was fully exploited to accommodate various diamines and α-ketoesters for the efficient synthesis of chiral 3,4-dihydroquinoxalinones.
中文翻译:

1,2-二胺作为酰胺化和铑催化不对称还原胺化级联反应中的胺源
1,2-二胺和过渡金属的牢固螯合会延迟甚至中断常规催化循环。在二胺和酮酯的酰胺化和不对称还原胺化 (ARA) 级联反应中,我们部署了多组添加剂,以确保由铑和多功能且高度模块化的亚磷酰胺-膦配体的配合物催化的平稳转化。充分利用配体的可调性来适应各种二胺和α-酮酯,从而有效合成手性 3,4-二氢喹喔啉酮。
更新日期:2022-08-02
中文翻译:

1,2-二胺作为酰胺化和铑催化不对称还原胺化级联反应中的胺源
1,2-二胺和过渡金属的牢固螯合会延迟甚至中断常规催化循环。在二胺和酮酯的酰胺化和不对称还原胺化 (ARA) 级联反应中,我们部署了多组添加剂,以确保由铑和多功能且高度模块化的亚磷酰胺-膦配体的配合物催化的平稳转化。充分利用配体的可调性来适应各种二胺和α-酮酯,从而有效合成手性 3,4-二氢喹喔啉酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号