当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-Assembly Modification of Head-to-Tail Cyclic Peptides by Methionine-Directed β-C(sp3)–H Arylation
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.orglett.2c02253 Gang Li 1 , Feipeng Yuan 1 , Bo Yao 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.orglett.2c02253 Gang Li 1 , Feipeng Yuan 1 , Bo Yao 1, 2
Affiliation
|
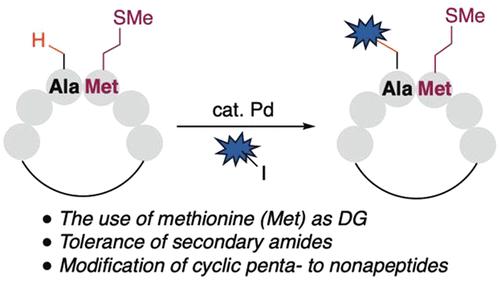
Peptide modification by C(sp3)–H functionalization of internal residues remains a major challenge due to the inhibitory effect of peptide bonds. In this work, we developed a methionine-directed β-C(sp3)–H arylation method for internal alanine functionalization. By tuning the σC–C bond rotation of internal Ala through head-to-tail cyclization, we overcame the inhibitory effect and functionalized a wide range of head-to-tail cyclic peptides with aryl iodides with excellent position selectivity.
中文翻译:

蛋氨酸定向 β-C(sp3)–H 芳基化对头尾环肽的组装后修饰
由于肽键的抑制作用,通过内部残基的 C(sp 3 )-H 官能化进行肽修饰仍然是一项重大挑战。在这项工作中,我们开发了一种以蛋氨酸为导向的 β-C(sp 3 )-H 芳基化方法,用于内部丙氨酸功能化。通过头尾环化调整内部 Ala 的 σ C-C键旋转,我们克服了抑制效应,并用芳基碘化物对各种头尾环肽进行了功能化,具有出色的位置选择性。
更新日期:2022-08-02
中文翻译:

蛋氨酸定向 β-C(sp3)–H 芳基化对头尾环肽的组装后修饰
由于肽键的抑制作用,通过内部残基的 C(sp 3 )-H 官能化进行肽修饰仍然是一项重大挑战。在这项工作中,我们开发了一种以蛋氨酸为导向的 β-C(sp 3 )-H 芳基化方法,用于内部丙氨酸功能化。通过头尾环化调整内部 Ala 的 σ C-C键旋转,我们克服了抑制效应,并用芳基碘化物对各种头尾环肽进行了功能化,具有出色的位置选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号