当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct H2O2 Synthesis, without H2 Gas
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-02 , DOI: 10.1021/jacs.2c03158 Aoxue Huang 1 , Roxanna S Delima 2, 3 , Yongwook Kim 1 , Eric W Lees 3 , Fraser G L Parlane 1, 2 , David J Dvorak 2 , Michael B Rooney 1 , Ryan P Jansonius 1 , Arthur G Fink 1 , Zishuai Zhang 1 , Curtis P Berlinguette 1, 2, 3, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-02 , DOI: 10.1021/jacs.2c03158 Aoxue Huang 1 , Roxanna S Delima 2, 3 , Yongwook Kim 1 , Eric W Lees 3 , Fraser G L Parlane 1, 2 , David J Dvorak 2 , Michael B Rooney 1 , Ryan P Jansonius 1 , Arthur G Fink 1 , Zishuai Zhang 1 , Curtis P Berlinguette 1, 2, 3, 4
Affiliation
|
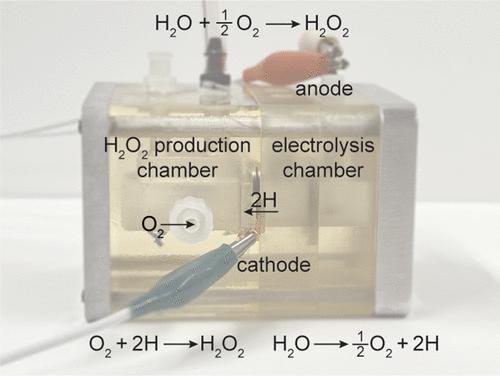
We report here the direct hydrogenation of O2 gas to form hydrogen peroxide (H2O2) using a membrane reactor without H2 gas. Hydrogen is sourced from water, and the reactor is driven by electricity. Hydrogenation chemistry is achieved using a hydrogen-permeable Pd foil that separates an electrolysis chamber that generates reactive H atoms, from a hydrogenation chamber where H atoms react with O2 to form H2O2. Our results show that the concentration of H2O2 can be increased ∼8 times (from 56.5 to 443 mg/L) by optimizing the ratio of methanol-to-water in the chemical chamber, and through catalyst design. We demonstrate that the concentration of H2O2 is acutely sensitive to the H2O2 decomposition rate. This decomposition rate can be minimized by using AuPd alloy catalysts instead of pure Pd. This study presents a new pathway to directly synthesize H2O2 using water electrolysis without ever using H2 gas.
中文翻译:

直接 H2O2 合成,无需 H2 气
我们在此报告了使用没有 H 2气体的膜反应器将 O 2气体直接加氢以形成过氧化氢 (H 2 O 2 ) 。氢气来自水,反应堆由电力驱动。加氢化学是使用透氢 Pd 箔实现的,该箔将产生反应性 H 原子的电解室与 H 原子与 O 2反应形成 H 2 O 2的加氢室隔开。我们的结果表明,H 2 O 2的浓度通过优化化学室中甲醇与水的比例以及催化剂设计,可以将其提高 8 倍(从 56.5 到 443 mg/L)。我们证明了H 2 O 2 的浓度对H 2 O 2分解速率非常敏感。通过使用 AuPd 合金催化剂而不是纯 Pd,可以将这种分解速率降至最低。本研究提出了一种使用水电解直接合成 H 2 O 2而无需使用 H 2气体的新途径。
更新日期:2022-08-02
中文翻译:

直接 H2O2 合成,无需 H2 气
我们在此报告了使用没有 H 2气体的膜反应器将 O 2气体直接加氢以形成过氧化氢 (H 2 O 2 ) 。氢气来自水,反应堆由电力驱动。加氢化学是使用透氢 Pd 箔实现的,该箔将产生反应性 H 原子的电解室与 H 原子与 O 2反应形成 H 2 O 2的加氢室隔开。我们的结果表明,H 2 O 2的浓度通过优化化学室中甲醇与水的比例以及催化剂设计,可以将其提高 8 倍(从 56.5 到 443 mg/L)。我们证明了H 2 O 2 的浓度对H 2 O 2分解速率非常敏感。通过使用 AuPd 合金催化剂而不是纯 Pd,可以将这种分解速率降至最低。本研究提出了一种使用水电解直接合成 H 2 O 2而无需使用 H 2气体的新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号