当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Based Design of Stapled Peptides That Bind GABARAP and Inhibit Autophagy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-02 , DOI: 10.1021/jacs.2c04699 Hawley Brown 1 , Mia Chung 1 , Alina Üffing 2, 3 , Nefeli Batistatou 1 , Tiffany Tsang 1 , Samantha Doskocil 4 , Weiqun Mao 4 , Dieter Willbold 2, 3 , Robert C Bast 4 , Zhen Lu 4 , Oliver H Weiergräber 2 , Joshua A Kritzer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-02 , DOI: 10.1021/jacs.2c04699 Hawley Brown 1 , Mia Chung 1 , Alina Üffing 2, 3 , Nefeli Batistatou 1 , Tiffany Tsang 1 , Samantha Doskocil 4 , Weiqun Mao 4 , Dieter Willbold 2, 3 , Robert C Bast 4 , Zhen Lu 4 , Oliver H Weiergräber 2 , Joshua A Kritzer 1
Affiliation
|
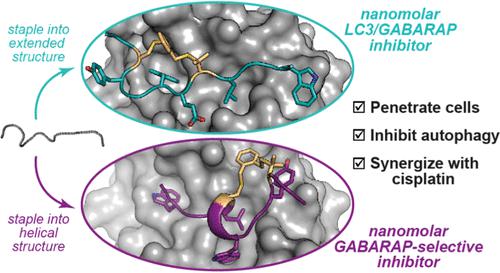
The LC3/GABARAP family of proteins is involved in nearly every stage of autophagy. Inhibition of LC3/GABARAP proteins is a promising approach to blocking autophagy, which sensitizes advanced cancers to DNA-damaging chemotherapy. Here, we report the structure-based design of stapled peptides that inhibit GABARAP with nanomolar affinities. Small changes in staple structure produced stapled peptides with very different binding modes and functional differences in LC3/GABARAP paralog selectivity, ranging from highly GABARAP-specific to broad inhibition of both subfamilies. The stapled peptides exhibited considerable cytosolic penetration and resistance to biological degradation. They also reduced autophagic flux in cultured ovarian cancer cells and sensitized ovarian cancer cells to cisplatin. These small, potent stapled peptides represent promising autophagy-modulating compounds that can be developed as novel cancer therapeutics and novel mediators of targeted protein degradation.
中文翻译:

基于结构的结合 GABARAP 并抑制自噬的缝合肽的设计
LC3/GABARAP 蛋白家族几乎参与自噬的每个阶段。抑制 LC3/GABARAP 蛋白是一种很有前途的阻断自噬的方法,自噬使晚期癌症对 DNA 损伤性化疗敏感。在这里,我们报告了基于结构的钉合肽设计,该肽以纳摩尔亲和力抑制 GABARAP。钉合结构的微小变化产生了具有非常不同的结合模式和 LC3/GABARAP 旁系同源选择性功能差异的钉合肽,范围从高度 GABARAP 特异性到对两个亚家族的广泛抑制。钉合的肽表现出相当大的细胞溶质渗透性和对生物降解的抵抗力。他们还减少了培养的卵巢癌细胞的自噬通量,并使卵巢癌细胞对顺铂敏感。这些小而有效的钉合肽代表了有前途的自噬调节化合物,可以开发为新型癌症治疗剂和靶向蛋白质降解的新型介质。
更新日期:2022-08-02
中文翻译:

基于结构的结合 GABARAP 并抑制自噬的缝合肽的设计
LC3/GABARAP 蛋白家族几乎参与自噬的每个阶段。抑制 LC3/GABARAP 蛋白是一种很有前途的阻断自噬的方法,自噬使晚期癌症对 DNA 损伤性化疗敏感。在这里,我们报告了基于结构的钉合肽设计,该肽以纳摩尔亲和力抑制 GABARAP。钉合结构的微小变化产生了具有非常不同的结合模式和 LC3/GABARAP 旁系同源选择性功能差异的钉合肽,范围从高度 GABARAP 特异性到对两个亚家族的广泛抑制。钉合的肽表现出相当大的细胞溶质渗透性和对生物降解的抵抗力。他们还减少了培养的卵巢癌细胞的自噬通量,并使卵巢癌细胞对顺铂敏感。这些小而有效的钉合肽代表了有前途的自噬调节化合物,可以开发为新型癌症治疗剂和靶向蛋白质降解的新型介质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号