当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Regio- and Stereoselective Coupling of Alkynylsulfones with Alkenes: Access to Dichlorinated Vinyl Sulfones
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.orglett.2c02324 Dandan He 1, 2 , Wentao Zhong 1 , Miaomiao Zhou 3 , Bowen Wang 1 , Meng Li 1 , Huanfeng Jiang 1 , Wanqing Wu 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.orglett.2c02324 Dandan He 1, 2 , Wentao Zhong 1 , Miaomiao Zhou 3 , Bowen Wang 1 , Meng Li 1 , Huanfeng Jiang 1 , Wanqing Wu 1
Affiliation
|
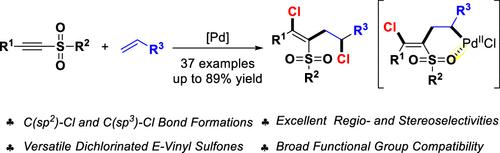
A palladium-catalyzed coupling reaction of alkynylsulfones with alkenes has been described, which provides an efficient and practical entry to various functionalized dichlorinated vinyl sulfones. This method features excellent regio- and stereoselectivities, good functional group compatibility, as well as mild reaction conditions. Mechanistic studies suggest that the reaction goes through sequential syn-chloropalladation, alkene insertion, and C(sp3)–Cl bond formation processes, and the sulfonyl group is crucial to the stereoselectivity control of the reaction.
中文翻译:

炔基砜与烯烃的钯催化区域选择性和立体选择性偶联:获得二氯乙烯基砜
已经描述了炔基砜与烯烃的钯催化偶联反应,这为各种官能化二氯化乙烯基砜提供了有效和实用的途径。该方法具有优异的区域和立体选择性、良好的官能团相容性以及温和的反应条件。机理研究表明,该反应经历了顺序的顺式氯钯化、烯烃插入和 C(sp 3 )-Cl 键形成过程,磺酰基对反应的立体选择性控制至关重要。
更新日期:2022-08-02
中文翻译:

炔基砜与烯烃的钯催化区域选择性和立体选择性偶联:获得二氯乙烯基砜
已经描述了炔基砜与烯烃的钯催化偶联反应,这为各种官能化二氯化乙烯基砜提供了有效和实用的途径。该方法具有优异的区域和立体选择性、良好的官能团相容性以及温和的反应条件。机理研究表明,该反应经历了顺序的顺式氯钯化、烯烃插入和 C(sp 3 )-Cl 键形成过程,磺酰基对反应的立体选择性控制至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号