Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Study of NO Adsorption by Hydroxyl-Containing Char with the Participation of Na/K
Langmuir ( IF 3.7 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.langmuir.2c01244 Long Chen 1 , Jiancheng Yang 1, 2 , Mingkai Zhang 1 , Mengkai Gao 1 , Jiachun Su 1 , Yuan Huang 1 , Zhikun Zhang 1, 2 , Zhuozhi Wang 3 , Lianfei Xu 1 , Boxiong Shen 1, 2, 3
Langmuir ( IF 3.7 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.langmuir.2c01244 Long Chen 1 , Jiancheng Yang 1, 2 , Mingkai Zhang 1 , Mengkai Gao 1 , Jiachun Su 1 , Yuan Huang 1 , Zhikun Zhang 1, 2 , Zhuozhi Wang 3 , Lianfei Xu 1 , Boxiong Shen 1, 2, 3
Affiliation
|
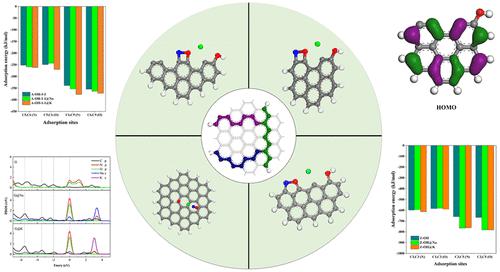
The study of the effects of Na and K on the heterogeneous adsorption of hydroxyl-containing char with NO is important for the clean utilization of high alkali coal. In this paper, the effects of Na/K atoms on the adsorption of NO on the char surface were investigated at the GGA-PBE level by choosing zigzag type, armchair type, and saturated hydroxyl-containing char structures based on DFT. It was found that the adsorption stability of NO on structures with active sites was greater for sites close to the hydroxyl group than that for sites far from the hydroxyl group. The stability of char doped by Na/K is related to the char structure and the position of functional groups. The most stable Na/K doped structures are Z-OH-2 (Eads= −350.50 kJ/mol) and A-OH-1-2 (Eads= −339.17 kJ/mol), respectively. The participation of Na/K can increase the adsorption energy of the three structures with NO, and especially the adsorption energy of saturated char with NO is increased by as much as 5 times. The reason for that is the promotion of the hybridization of the C and NO p orbitals. The comprehensive analysis of electrostatic potential, charge transfer, and front orbitals indicates that the effects of decorated sodium and potassium atoms on the char surface are very similar. This study lays a theoretical foundation for the study of the heterogeneous reduction process.
中文翻译:

Na/K参与含羟基炭吸附NO的理论研究
研究Na和K对含羟基焦炭与NO的非均相吸附的影响,对高碱煤的清洁利用具有重要意义。本文在 GGA-PBE 水平上,基于 DFT 选择锯齿型、扶手椅型和饱和含羟基的炭结构,研究了 Na/K 原子对炭表面 NO 吸附的影响。研究发现,靠近羟基的位点比远离羟基的位点对具有活性位点的结构的吸附稳定性更高。Na/K掺杂炭的稳定性与炭的结构和官能团的位置有关。最稳定的 Na/K 掺杂结构是 Z-OH-2 ( E ads = -350.50 kJ/mol) 和 A-OH-1-2 ( E ads= -339.17 kJ/mol),分别。Na/K的参与可以提高三种结构对NO的吸附能,尤其是饱和炭对NO的吸附能提高了5倍之多。其原因是促进了 C 和 NO p 轨道的杂化。对静电势、电荷转移和前轨道的综合分析表明,修饰的钠和钾原子对炭表面的影响非常相似。本研究为异质还原过程的研究奠定了理论基础。
更新日期:2022-08-02
中文翻译:

Na/K参与含羟基炭吸附NO的理论研究
研究Na和K对含羟基焦炭与NO的非均相吸附的影响,对高碱煤的清洁利用具有重要意义。本文在 GGA-PBE 水平上,基于 DFT 选择锯齿型、扶手椅型和饱和含羟基的炭结构,研究了 Na/K 原子对炭表面 NO 吸附的影响。研究发现,靠近羟基的位点比远离羟基的位点对具有活性位点的结构的吸附稳定性更高。Na/K掺杂炭的稳定性与炭的结构和官能团的位置有关。最稳定的 Na/K 掺杂结构是 Z-OH-2 ( E ads = -350.50 kJ/mol) 和 A-OH-1-2 ( E ads= -339.17 kJ/mol),分别。Na/K的参与可以提高三种结构对NO的吸附能,尤其是饱和炭对NO的吸附能提高了5倍之多。其原因是促进了 C 和 NO p 轨道的杂化。对静电势、电荷转移和前轨道的综合分析表明,修饰的钠和钾原子对炭表面的影响非常相似。本研究为异质还原过程的研究奠定了理论基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号