当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ/Operando Spectroscopic Studies on the NH3–SCR Mechanism over Fe–Zeolites
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-02 , DOI: 10.1021/acscatal.2c02904 Shunsaku Yasumura 1 , Yucheng Qian 1 , Taisetsu Kato 1 , Shinya Mine 1 , Takashi Toyao 1 , Zen Maeno 2 , Ken-ichi Shimizu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-08-02 , DOI: 10.1021/acscatal.2c02904 Shunsaku Yasumura 1 , Yucheng Qian 1 , Taisetsu Kato 1 , Shinya Mine 1 , Takashi Toyao 1 , Zen Maeno 2 , Ken-ichi Shimizu 1
Affiliation
|
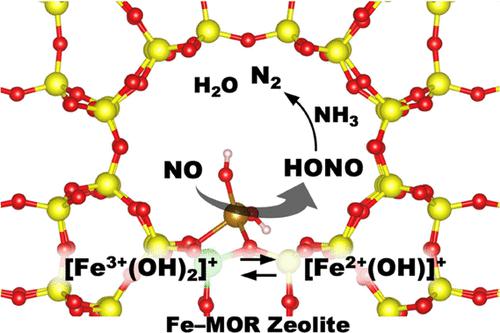
Reduction/oxidation half-cycles of the selective catalytic reduction of NO with NH3 (NH3–SCR) over Fe-exchanged mordenite (MOR) zeolites at 300 °C were investigated by in situ/operando spectroscopy (infrared, UV–vis, and Fe K-edge X-ray absorption near edge structure) and density functional theory (DFT) calculation. The reduction of Fe3+ into Fe2+ and the simultaneous formation of N2 and H2O in the reduction half-cycle (under NO + NH3) were demonstrated by different spectroscopic results. In the subsequent oxidation half-cycle (under O2 or NO + O2), Fe2+ was reoxidized into Fe3+. The reduction half-cycle comprises several elementary steps. Reduction of Fe3+–OH by NO producing Fe2+ and NO+ species was observed at low temperatures (<100 °C), while N2 formation due to the reduction of NO+ was observed under subsequent NH3 exposure at 100 °C. Under transient conditions, NH3 on Brønsted acid sites (B–NH3) reacted with NO to generate N2 when the coverage of B–NH3 was low, indicating that B–NH3 is not a spectator but a reservoir of NH3. Transition state calculation theoretically suggested that the formation of nitrous acid (HONO) intermediates from [Fe3+(OH–)2]+ at a Al site and gaseous NO was a facile process (Ea = 29.2 kJ/mol). Combining the experimental observation and DFT calculation, the mechanism of the reduction half-cycle over Fe–zeolites was proposed; [Fe3+(OH–)2]+ is reduced by NO to produce a HONO intermediate, which then reacts with NH3 on Brønsted acid sites to yield H2O and N2 via NO+ species. Based on the mechanistic insights above, Fe–zeolites (MOR and β) with different Fe loadings and Si/Al ratios were tested for NH3–SCR reaction. Consequently, 2.7 wt % Fe-loaded zeolites with a relatively large number of Brønsted acid sites (Al-rich β with a Si/Al ratio of 5) showed the highest NOx conversion in a low-temperature region.
中文翻译:

Fe-沸石上NH3-SCR机理的原位/操作光谱研究
通过原位/操作光谱(红外、紫外-可见、和Fe K-edge X射线吸收近边缘结构)和密度泛函理论(DFT)计算。不同的光谱结果证明了在还原半循环中(在NO + NH 3下) Fe 3+还原成Fe 2+和同时形成N 2和H 2 O。在随后的氧化半循环中(在 O 2或 NO + O 2下),Fe2+被再氧化成Fe 3+。还原半周期包括几个基本步骤。在低温(<100 °C)观察到通过 NO还原 Fe 3+ –OH 产生 Fe 2+和 NO +物质,而在随后的 NH 3暴露于 100 °下观察到由于 NO +的还原而形成 N 2 C。在瞬态条件下,当 B-NH 3的覆盖率较低时,布朗斯台德酸位点 (B-NH 3 ) 上的 NH 3与 NO 反应生成 N 2,表明 B-NH 3不是旁观者,而是 NH 3的储库. 过渡态计算理论上表明,从 [Fe 3+ (OH – ) 2 ] +在 Al 位点和气态 NO形成亚硝酸 (HONO) 中间体是一个容易的过程( E a = 29.2 kJ/mol)。结合实验观察和DFT计算,提出了Fe-沸石还原半循环的机理;[Fe 3+ (OH – ) 2 ] +被 NO 还原生成 HONO 中间体,然后与 NH 3在布朗斯台德酸位点反应生成 H 2 O 和 N 2通过 NO +物种。基于上述机理见解,测试了具有不同 Fe 负载量和 Si/Al 比的 Fe-沸石(MOR 和 β)用于 NH 3 -SCR 反应。因此,具有相对大量布朗斯台德酸位点的 2.7 wt% Fe 负载沸石(富含 Al 的 β,Si/Al 比为 5)在低温区域显示出最高的 NO x转化率。
更新日期:2022-08-02
中文翻译:

Fe-沸石上NH3-SCR机理的原位/操作光谱研究
通过原位/操作光谱(红外、紫外-可见、和Fe K-edge X射线吸收近边缘结构)和密度泛函理论(DFT)计算。不同的光谱结果证明了在还原半循环中(在NO + NH 3下) Fe 3+还原成Fe 2+和同时形成N 2和H 2 O。在随后的氧化半循环中(在 O 2或 NO + O 2下),Fe2+被再氧化成Fe 3+。还原半周期包括几个基本步骤。在低温(<100 °C)观察到通过 NO还原 Fe 3+ –OH 产生 Fe 2+和 NO +物质,而在随后的 NH 3暴露于 100 °下观察到由于 NO +的还原而形成 N 2 C。在瞬态条件下,当 B-NH 3的覆盖率较低时,布朗斯台德酸位点 (B-NH 3 ) 上的 NH 3与 NO 反应生成 N 2,表明 B-NH 3不是旁观者,而是 NH 3的储库. 过渡态计算理论上表明,从 [Fe 3+ (OH – ) 2 ] +在 Al 位点和气态 NO形成亚硝酸 (HONO) 中间体是一个容易的过程( E a = 29.2 kJ/mol)。结合实验观察和DFT计算,提出了Fe-沸石还原半循环的机理;[Fe 3+ (OH – ) 2 ] +被 NO 还原生成 HONO 中间体,然后与 NH 3在布朗斯台德酸位点反应生成 H 2 O 和 N 2通过 NO +物种。基于上述机理见解,测试了具有不同 Fe 负载量和 Si/Al 比的 Fe-沸石(MOR 和 β)用于 NH 3 -SCR 反应。因此,具有相对大量布朗斯台德酸位点的 2.7 wt% Fe 负载沸石(富含 Al 的 β,Si/Al 比为 5)在低温区域显示出最高的 NO x转化率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号