Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel extraction route of lithium from α-spodumene by dry chlorination
RSC Advances ( IF 3.9 ) Pub Date : 2022-08-02 , DOI: 10.1039/d2ra03233c Allen Yushark Fosu 1 , Ndue Kanari 1 , Danièle Bartier 1 , James Vaughan 2 , Alexandre Chagnes 1
RSC Advances ( IF 3.9 ) Pub Date : 2022-08-02 , DOI: 10.1039/d2ra03233c Allen Yushark Fosu 1 , Ndue Kanari 1 , Danièle Bartier 1 , James Vaughan 2 , Alexandre Chagnes 1
Affiliation
|
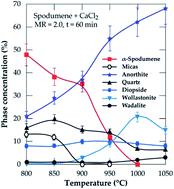
Processing spodumene for lithium is challenging as it requires a high temperature transformation of the natural α-monoclinic form to β-tetragonal form, usually followed by acid baking and digestion. This three-step extraction process requires significant heat energy, acid, process complexity and residence time, leading to both operating and capital costs. An approach which helps to eliminate this challenge will therefore be a milestone in processing spodumene. This study, thus, investigates a direct chlorination of α-spodumene using calcium chloride followed by water leaching of the residue to recover lithium, which reduces the energy requirement and number of unit operations. HSC Chemistry software was used to simulate the process using both phases (α and β) of the mineral up to 1100 °C prior to experimental investigation. The α-form was the only polymorph identified in residues after leaching, suggesting that the extraction is directly from the α-phase. However, an initial formation of a metastable β-form followed by a fast synthesis of lithium chloride from it is also suspected. Under optimal conditions of calcium chloride/spodumene molar ratio of 2.0, and 1000 °C treatment for 60 minutes, almost 90 percent lithium chloride was extracted and 85 percent was recovered to the leach solution with the remainder exiting with the off-gas. An apparent activation energy of about 122 ± 6 kJ mol−1 was obtained at temperatures ranging from 800 to 950 °C during the process.
中文翻译:

干氯化法从α-锂辉石中提取锂的新路线
锂锂辉石的加工具有挑战性,因为它需要将天然 α-单斜晶型高温转变为 β-四方晶型,通常随后进行酸烘烤和消化。这种三步萃取工艺需要大量的热能、酸、工艺复杂性和停留时间,从而导致运营和资本成本。因此,有助于消除这一挑战的方法将成为处理锂辉石的里程碑。因此,本研究研究了使用氯化钙直接氯化 α-锂辉石,然后对残渣进行水浸以回收锂,从而减少能源需求和单元操作数量。在实验研究之前,HSC 化学软件用于模拟使用矿物的两个相(α 和 β)的过程,温度高达 1100 °C。α-型是浸出后残留物中唯一鉴定出的多晶型物,这表明提取物是直接从 α-相中提取的。然而,也怀疑最初形成亚稳态 β 型,然后从中快速合成氯化锂。在氯化钙/锂辉石摩尔比为 2.0 和 1000°C 处理 60 分钟的最佳条件下,几乎 90% 的氯化锂被提取出来,85% 的氯化锂被回收到浸出液中,其余的随废气排出。表观活化能约为 122 ± 6 kJ mol 在氯化钙/锂辉石摩尔比为 2.0 和 1000°C 处理 60 分钟的最佳条件下,几乎 90% 的氯化锂被提取出来,85% 的氯化锂被回收到浸出液中,其余的随废气排出。表观活化能约为 122 ± 6 kJ mol 在氯化钙/锂辉石摩尔比为 2.0 和 1000°C 处理 60 分钟的最佳条件下,几乎 90% 的氯化锂被提取出来,85% 的氯化锂被回收到浸出液中,其余的随废气排出。表观活化能约为 122 ± 6 kJ mol在此过程中,在 800 至 950 °C 的温度范围内获得-1 。
更新日期:2022-08-02
中文翻译:

干氯化法从α-锂辉石中提取锂的新路线
锂锂辉石的加工具有挑战性,因为它需要将天然 α-单斜晶型高温转变为 β-四方晶型,通常随后进行酸烘烤和消化。这种三步萃取工艺需要大量的热能、酸、工艺复杂性和停留时间,从而导致运营和资本成本。因此,有助于消除这一挑战的方法将成为处理锂辉石的里程碑。因此,本研究研究了使用氯化钙直接氯化 α-锂辉石,然后对残渣进行水浸以回收锂,从而减少能源需求和单元操作数量。在实验研究之前,HSC 化学软件用于模拟使用矿物的两个相(α 和 β)的过程,温度高达 1100 °C。α-型是浸出后残留物中唯一鉴定出的多晶型物,这表明提取物是直接从 α-相中提取的。然而,也怀疑最初形成亚稳态 β 型,然后从中快速合成氯化锂。在氯化钙/锂辉石摩尔比为 2.0 和 1000°C 处理 60 分钟的最佳条件下,几乎 90% 的氯化锂被提取出来,85% 的氯化锂被回收到浸出液中,其余的随废气排出。表观活化能约为 122 ± 6 kJ mol 在氯化钙/锂辉石摩尔比为 2.0 和 1000°C 处理 60 分钟的最佳条件下,几乎 90% 的氯化锂被提取出来,85% 的氯化锂被回收到浸出液中,其余的随废气排出。表观活化能约为 122 ± 6 kJ mol 在氯化钙/锂辉石摩尔比为 2.0 和 1000°C 处理 60 分钟的最佳条件下,几乎 90% 的氯化锂被提取出来,85% 的氯化锂被回收到浸出液中,其余的随废气排出。表观活化能约为 122 ± 6 kJ mol在此过程中,在 800 至 950 °C 的温度范围内获得-1 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号