当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Migrative Carbofluorination of Saturated Amides Enabled by Pd-Based Dyotropic Rearrangement
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-02 , DOI: 10.1021/jacs.2c06578 Guoqiang Yang 1 , Hua Wu 1, 2 , Simone Gallarati 3 , Clémence Corminboeuf 3 , Qian Wang 1 , Jieping Zhu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-02 , DOI: 10.1021/jacs.2c06578 Guoqiang Yang 1 , Hua Wu 1, 2 , Simone Gallarati 3 , Clémence Corminboeuf 3 , Qian Wang 1 , Jieping Zhu 1
Affiliation
|
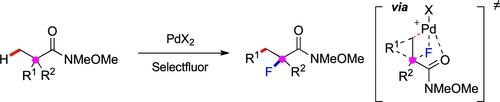
Directly editing an all-carbon quaternary carbon itself of nonstrained acyclic molecules remains underexploited despite the recent advances in the fields of both C–H and C–C bond activation. Herein, we report a palladium-catalyzed migrative carbofluorination of saturated amides enabled by the activation of both the C(sp3)–H and the Cquaternary–Cσ bonds. In this transformation, the α-quaternary carbon of Weinreb amides is converted to α-tertiary fluoride with concurrent migration of an aryl or an amido group from the α- to β-carbon. DFT calculations indicate that the dyotropic rearrangement proceeds through an unusual anti-selective [2.1.0] bicyclic transition state. The reaction, compatible with a broad range of functional groups, is stereospecific and is applicable to the synthesis of enantioenriched products.
中文翻译:

基于 Pd 的电致重排实现的饱和酰胺的迁移碳氟化
尽管最近在 C-H 和 C-C 键活化领域取得了进展,但直接编辑非应变无环分子的全碳季碳本身仍未得到充分利用。在此,我们报告了通过激活 C(sp 3 )-H 和 C季-Cσ 键实现的钯催化的饱和酰胺的迁移碳氟化反应。在这种转变中,Weinreb 酰胺的 α-季碳转化为 α-叔氟化物,同时芳基或酰胺基从 α-碳迁移到 β-碳。DFT 计算表明,电致重排通过一个不寻常的反-选择性[2.1.0]双环过渡态。该反应与多种官能团相容,具有立体专一性,适用于对映体富集产物的合成。
更新日期:2022-08-02
中文翻译:

基于 Pd 的电致重排实现的饱和酰胺的迁移碳氟化
尽管最近在 C-H 和 C-C 键活化领域取得了进展,但直接编辑非应变无环分子的全碳季碳本身仍未得到充分利用。在此,我们报告了通过激活 C(sp 3 )-H 和 C季-Cσ 键实现的钯催化的饱和酰胺的迁移碳氟化反应。在这种转变中,Weinreb 酰胺的 α-季碳转化为 α-叔氟化物,同时芳基或酰胺基从 α-碳迁移到 β-碳。DFT 计算表明,电致重排通过一个不寻常的反-选择性[2.1.0]双环过渡态。该反应与多种官能团相容,具有立体专一性,适用于对映体富集产物的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号