当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling the Capacitive Charge Storage Mechanism of Nitrogen-Doped Porous Carbons by EQCM and ssNMR
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-01 , DOI: 10.1021/jacs.2c04841 En Zhang 1 , Yih-Chyng Wu 2, 3 , Hui Shao 2, 3 , Vytautas Klimavicius 4, 5 , Hanyue Zhang 1 , Pierre-Louis Taberna 2 , Julia Grothe 1 , Gerd Buntkowsky 5 , Fei Xu 1 , Patrice Simon 2, 3 , Stefan Kaskel 1, 6
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-01 , DOI: 10.1021/jacs.2c04841 En Zhang 1 , Yih-Chyng Wu 2, 3 , Hui Shao 2, 3 , Vytautas Klimavicius 4, 5 , Hanyue Zhang 1 , Pierre-Louis Taberna 2 , Julia Grothe 1 , Gerd Buntkowsky 5 , Fei Xu 1 , Patrice Simon 2, 3 , Stefan Kaskel 1, 6
Affiliation
|
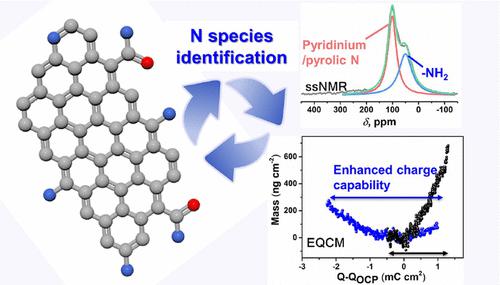
Fundamental understanding of ion electroadsorption processes in porous electrodes on a molecular level provides important guidelines for next-generation energy storage devices like electric double layer capacitors (EDLCs). Porous carbons functionalized by heteroatoms show enhanced capacitive performance, but the underlying mechanism is still elusive, due to the lack of reliable tools to precisely identify multiple N species and establish clear structure property relations. Here, we use advanced analytical techniques such as low-temperature solid-state NMR (ssNMR) and electrochemical quartz crystal microbalance (EQCM) to relate the complex nitrogen functionalities to the charging mechanisms and capacitive performance. For the first time, it is demonstrated at a molecular level that N-doping strongly influences the electroadsorption mechanism in EDLCs. Without N-doping, anion (SO42–) adsorption–desorption dominates the charging mechanism, whereas after doping, Li+ electroadsorption plays a key role. With the help of EQCM, it is demonstrated that SO42– is strongly immobilized on the N-doped surface, leaving Li+ as the main charge carrier. The smaller size and higher concentration of Li+ compared to SO42– benefit a higher capacitance. Amine/amide N is responsible for high capacitance, but surprisingly the pyridinic, pyrrolic, and graphitic N groups have no significant influence. 2D 1H-15N NMR spectroscopy indicates that the conversion from pyridinium to pyrrolic N gives rise to a slightly decreased capacitance. This work not only demonstrates ssNMR as a powerful tool for surface chemistry characterization of electrode materials but also uncovers the related charging mechanism by EQCM, paving the way toward a comprehensive picture of EDLC chemistry.
中文翻译:

通过 EQCM 和 ssNMR 揭示氮掺杂多孔碳的电容性电荷存储机制
在分子水平上对多孔电极中离子电吸附过程的基本理解为下一代储能设备(如双电层电容器 (EDLC))提供了重要指导。由杂原子功能化的多孔碳显示出增强的电容性能,但由于缺乏可靠的工具来精确识别多种 N 物种并建立清晰的结构性质关系,其潜在机制仍然难以捉摸。在这里,我们使用先进的分析技术,如低温固态核磁共振 (ssNMR) 和电化学石英晶体微量天平 (EQCM),将复杂的氮功能与充电机制和电容性能联系起来。首次,在分子水平上证明了 N 掺杂强烈影响 EDLC 中的电吸附机制。在没有 N 掺杂的情况下,阴离子 (SO4 2- ) 吸附-解吸主导充电机制,而掺杂后,Li +电吸附起关键作用。在 EQCM 的帮助下,证明 SO 4 2-牢固地固定在 N 掺杂表面上,而 Li +成为主要的电荷载流子。与 SO 4 2-相比,Li +的较小尺寸和较高浓度有利于较高的电容。胺/酰胺 N 负责高电容,但令人惊讶的是,吡啶、吡咯和石墨 N 基团没有显着影响。2D 1 H- 15N NMR 光谱表明,从吡啶鎓到吡咯 N 的转化导致电容略有下降。这项工作不仅证明了 ssNMR 作为电极材料表面化学表征的强大工具,而且还揭示了 EQCM 的相关充电机制,为全面了解 EDLC 化学铺平了道路。
更新日期:2022-08-01
中文翻译:

通过 EQCM 和 ssNMR 揭示氮掺杂多孔碳的电容性电荷存储机制
在分子水平上对多孔电极中离子电吸附过程的基本理解为下一代储能设备(如双电层电容器 (EDLC))提供了重要指导。由杂原子功能化的多孔碳显示出增强的电容性能,但由于缺乏可靠的工具来精确识别多种 N 物种并建立清晰的结构性质关系,其潜在机制仍然难以捉摸。在这里,我们使用先进的分析技术,如低温固态核磁共振 (ssNMR) 和电化学石英晶体微量天平 (EQCM),将复杂的氮功能与充电机制和电容性能联系起来。首次,在分子水平上证明了 N 掺杂强烈影响 EDLC 中的电吸附机制。在没有 N 掺杂的情况下,阴离子 (SO4 2- ) 吸附-解吸主导充电机制,而掺杂后,Li +电吸附起关键作用。在 EQCM 的帮助下,证明 SO 4 2-牢固地固定在 N 掺杂表面上,而 Li +成为主要的电荷载流子。与 SO 4 2-相比,Li +的较小尺寸和较高浓度有利于较高的电容。胺/酰胺 N 负责高电容,但令人惊讶的是,吡啶、吡咯和石墨 N 基团没有显着影响。2D 1 H- 15N NMR 光谱表明,从吡啶鎓到吡咯 N 的转化导致电容略有下降。这项工作不仅证明了 ssNMR 作为电极材料表面化学表征的强大工具,而且还揭示了 EQCM 的相关充电机制,为全面了解 EDLC 化学铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号