当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C3-Selective Trifluoromethylthiolation and Difluoromethylthiolation of Pyridines and Pyridine Drugs via Dihydropyridine Intermediates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-01 , DOI: 10.1021/jacs.2c06776 Xin-Yue Zhou 1 , Ming Zhang 1 , Zhong Liu 1 , Jia-Hao He 1 , Xiao-Chen Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-08-01 , DOI: 10.1021/jacs.2c06776 Xin-Yue Zhou 1 , Ming Zhang 1 , Zhong Liu 1 , Jia-Hao He 1 , Xiao-Chen Wang 1
Affiliation
|
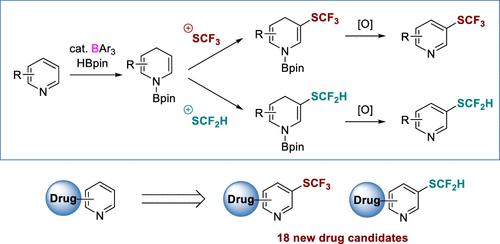
Herein, we report a method for C3-selective C–H tri- and difluoromethylthiolation of pyridines. The method relies on borane-catalyzed pyridine hydroboration for generation of nucleophilic dihydropyridines; these intermediates react with trifluoromethylthio and difluoromethylthio electrophiles to form functionalized dihydropyridines, which then undergo oxidative aromatization. The method can be used for late-stage functionalization of pyridine drugs for the generation of new drug candidates.
中文翻译:

通过二氢吡啶中间体对吡啶和吡啶类药物进行 C3-选择性三氟甲基硫醇化和二氟甲基硫醇化
在此,我们报告了一种 C3 选择性 C-H 三和二氟甲基硫醇化吡啶的方法。该方法依靠硼烷催化的吡啶硼氢化生成亲核二氢吡啶;这些中间体与三氟甲硫基和二氟甲硫基亲电子试剂反应形成功能化的二氢吡啶,然后进行氧化芳构化。该方法可用于吡啶类药物的后期功能化,以产生新的候选药物。
更新日期:2022-08-01
中文翻译:

通过二氢吡啶中间体对吡啶和吡啶类药物进行 C3-选择性三氟甲基硫醇化和二氟甲基硫醇化
在此,我们报告了一种 C3 选择性 C-H 三和二氟甲基硫醇化吡啶的方法。该方法依靠硼烷催化的吡啶硼氢化生成亲核二氢吡啶;这些中间体与三氟甲硫基和二氟甲硫基亲电子试剂反应形成功能化的二氢吡啶,然后进行氧化芳构化。该方法可用于吡啶类药物的后期功能化,以产生新的候选药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号