当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu(II)-Catalyzed Unsymmetrical Dioxidation of gem-Difluoroalkenes to Generate α,α-Difluorinated-α-phenoxyketones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-01 , DOI: 10.1021/acs.joc.2c00925 Suvajit Koley 1 , Kaylee T Cayton 2 , Gisela A González-Montiel 2 , M Ramu Yadav 3 , Douglas L Orsi 4 , Andrew J Intelli 1 , Paul Ha-Yeon Cheong 2 , Ryan A Altman 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-08-01 , DOI: 10.1021/acs.joc.2c00925 Suvajit Koley 1 , Kaylee T Cayton 2 , Gisela A González-Montiel 2 , M Ramu Yadav 3 , Douglas L Orsi 4 , Andrew J Intelli 1 , Paul Ha-Yeon Cheong 2 , Ryan A Altman 1
Affiliation
|
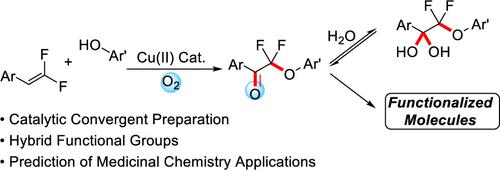
A Cu-based catalyst system convergently couples gem-difluoroalkenes with phenols under aerobic conditions to deliver α,α-difluorinated-α-phenoxyketones, an unstudied hybrid fluorinated functional group. Composed of α,α-difluorinated ketone and α,α-difluorinated ether moieties, these compounds have rarely been reported as a synthetic intermediate. Computational predictions and later experimental corroboration suggest that the phenoxy-substituted fluorinated ketone’s sp3-hybridized hydrate form is energetically favored relative to the respective nonether variant and that perturbation of the electronic character of the ketone can further encourage the formation of the hydrate. The more facile conversion between ketone and hydrate forms suggests that analogues should readily covalently inhibit proteases and other enzymes. Further functionalization of the ketone group enables access to other useful fluorinated functional groups.
中文翻译:

Cu(II) 催化偕二氟烯烃不对称二氧化生成 α,α-二氟化-α-苯氧基酮
铜基催化剂系统在有氧条件下将偕二氟烯烃与苯酚聚合偶联,生成α,α-二氟化-α-苯氧基酮,这是一种未经研究的杂化氟化官能团。这些化合物由α,α-二氟化酮和α,α-二氟化醚部分组成,很少被报道为合成中间体。计算预测和后来的实验证实表明,苯氧基取代的氟化酮的 sp 3杂化水合物形式相对于各自的非醚变体在能量上更有利,并且酮的电子特性的扰动可以进一步促进水合物的形成。酮和水合物形式之间更容易转化表明类似物应该容易共价抑制蛋白酶和其他酶。酮基团的进一步官能化能够获得其他有用的氟化官能团。
更新日期:2022-08-01
中文翻译:

Cu(II) 催化偕二氟烯烃不对称二氧化生成 α,α-二氟化-α-苯氧基酮
铜基催化剂系统在有氧条件下将偕二氟烯烃与苯酚聚合偶联,生成α,α-二氟化-α-苯氧基酮,这是一种未经研究的杂化氟化官能团。这些化合物由α,α-二氟化酮和α,α-二氟化醚部分组成,很少被报道为合成中间体。计算预测和后来的实验证实表明,苯氧基取代的氟化酮的 sp 3杂化水合物形式相对于各自的非醚变体在能量上更有利,并且酮的电子特性的扰动可以进一步促进水合物的形成。酮和水合物形式之间更容易转化表明类似物应该容易共价抑制蛋白酶和其他酶。酮基团的进一步官能化能够获得其他有用的氟化官能团。









































 京公网安备 11010802027423号
京公网安备 11010802027423号