Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A cyanide-catalyzed imino-Stetter reaction enables the concise total syntheses of rucaparib
RSC Advances ( IF 3.9 ) Pub Date : 2022-08-01 , DOI: 10.1039/d2ra03619c Jinjae Park 1 , Cheol-Hong Cheon 1
RSC Advances ( IF 3.9 ) Pub Date : 2022-08-01 , DOI: 10.1039/d2ra03619c Jinjae Park 1 , Cheol-Hong Cheon 1
Affiliation
|
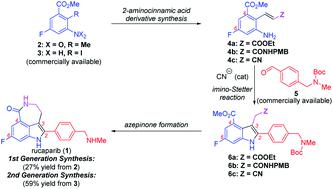
Two routes toward the synthesis of rucaparib, an FDA-approved drug used for the treatment of ovarian and prostate cancers, have been developed from commercially available starting materials utilizing the cyanide-catalyzed imino-Stetter reaction as the key step for the construction of the indole motif bearing all the desired substituents in their correct positions. In the first-generation synthesis, meta-fluorobenzoate, the starting material currently used in the process chemistry route of rucaparib, was converted into 4,6-disubstituted 2-aminocinnamic acid derivatives (ester or amide). The cyanide-catalyzed imino-Stetter reaction of aldimines derived from the resulting 2-aminocinnamic acid derivatives and a commercially available aldehyde afforded the desired indole-3-acetic acid derivatives. The final azepinone formation completed the total synthesis of rucaparib in 27% overall yield. To resolve the issues raised in the first-generation synthesis, we further developed a second-generation synthesis of rucaparib. The Heck reaction of a commercially available ortho-iodoaniline derivative with acrylonitrile provided 4,6-disubstituted 2-aminocinnamonitrile, which was subjected to the imino-Stetter reaction with the same aldehyde to provide the desired indole-3-acetonitrile product. Subsequent construction of the azepinone scaffold completed the total synthesis of rucaparib in 59% overall yield over three separation operations. The synthetic strategy reported herein can provide a highly practical route to access rucaparib from commercially available starting materials (5.2% overall yield in the current process chemistry route vs. 59% overall yield in the second-generation synthesis).
中文翻译:

氰化物催化的亚氨基-Stetter 反应实现了 rucaparib 的简洁全合成
rucaparib 是 FDA 批准的用于治疗卵巢癌和前列腺癌的药物,有两种合成路线,利用氰化物催化的亚氨基-Stetter 反应作为构建吲哚的关键步骤,从市售起始材料中开发出来基序在其正确位置上带有所有所需的取代基。在第一代合成中,目前rucaparib工艺化学路线中使用的起始原料间氟苯甲酸酯被转化为4,6-二取代的2-氨基肉桂酸衍生物(酯或酰胺)。由所得2-氨基肉桂酸衍生物衍生的醛亚胺与市售醛进行氰化物催化的亚氨基-Stetter反应,得到所需的吲哚-3-乙酸衍生物。最终的氮杂酮形成完成了 rucaparib 的全合成,总产率为 27%。为了解决第一代合成中出现的问题,我们进一步开发了rucaparib的第二代合成。市售的邻碘苯胺衍生物与丙烯腈进行Heck反应,得到4,6-二取代的2-氨基肉桂腈,将其与相同的醛进行亚氨基-Stetter反应,得到所需的吲哚-3-乙腈产物。随后构建的 azepinone 支架完成了 rucaparib 的全合成,经过 3 次分离操作,总产率为 59%。本文报道的合成策略可以提供一种高度实用的途径,从市售起始材料中获得rucaparib(当前工艺化学路线中的总产率为5.2%,而第二代合成中的总产率为59%)。
更新日期:2022-08-01
中文翻译:

氰化物催化的亚氨基-Stetter 反应实现了 rucaparib 的简洁全合成
rucaparib 是 FDA 批准的用于治疗卵巢癌和前列腺癌的药物,有两种合成路线,利用氰化物催化的亚氨基-Stetter 反应作为构建吲哚的关键步骤,从市售起始材料中开发出来基序在其正确位置上带有所有所需的取代基。在第一代合成中,目前rucaparib工艺化学路线中使用的起始原料间氟苯甲酸酯被转化为4,6-二取代的2-氨基肉桂酸衍生物(酯或酰胺)。由所得2-氨基肉桂酸衍生物衍生的醛亚胺与市售醛进行氰化物催化的亚氨基-Stetter反应,得到所需的吲哚-3-乙酸衍生物。最终的氮杂酮形成完成了 rucaparib 的全合成,总产率为 27%。为了解决第一代合成中出现的问题,我们进一步开发了rucaparib的第二代合成。市售的邻碘苯胺衍生物与丙烯腈进行Heck反应,得到4,6-二取代的2-氨基肉桂腈,将其与相同的醛进行亚氨基-Stetter反应,得到所需的吲哚-3-乙腈产物。随后构建的 azepinone 支架完成了 rucaparib 的全合成,经过 3 次分离操作,总产率为 59%。本文报道的合成策略可以提供一种高度实用的途径,从市售起始材料中获得rucaparib(当前工艺化学路线中的总产率为5.2%,而第二代合成中的总产率为59%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号