Redox Biology ( IF 10.7 ) Pub Date : 2022-07-30 , DOI: 10.1016/j.redox.2022.102415 Álvaro Viedma-Poyatos 1 , Patricia González-Jiménez 1 , María A Pajares 1 , Dolores Pérez-Sala 1
|
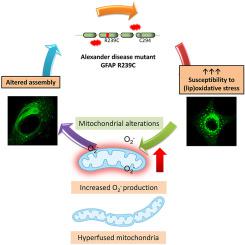
Alexander disease is a fatal neurological disorder caused by mutations in the intermediate filament protein Glial Fibrillary Acidic Protein (GFAP), which is key for astrocyte homeostasis. These mutations cause GFAP aggregation, astrocyte dysfunction and neurodegeneration. Remarkably, most of the known GFAP mutations imply a change by more nucleophilic amino acids, mainly cysteine or histidine, which are more susceptible to oxidation and lipoxidation. Therefore, we hypothesized that a higher susceptibility of Alexander disease GFAP mutants to oxidative or electrophilic damage, which frequently occurs during neurodegeneration, could contribute to disease pathogenesis. To address this point, we have expressed GFP-GFAP wild type or the harmful Alexander disease GFP-GFAP R239C mutant in astrocytic cells. Interestingly, GFAP R239C appears more oxidized than the wild type under control conditions, as indicated both by its lower cysteine residue accessibility and increased presence of disulfide-bonded oligomers. Moreover, GFP-GFAP R239C undergoes lipoxidation to a higher extent than GFAP wild type upon treatment with the electrophilic mediator 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2). Importantly, GFAP R239C filament organization is altered in untreated cells and is earlier and more severely disrupted than GFAP wild type upon exposure to oxidants (diamide, H2O2) or electrophiles (4-hydroxynonenal, 15d-PGJ2), which exacerbate GFAP R239C aggregation. Furthermore, H2O2 causes reversible alterations in GFAP wild type, but irreversible damage in GFAP R239C expressing cells. Finally, we show that GFAP R239C expression induces a more oxidized cellular status, with decreased free thiol content and increased mitochondrial superoxide generation. In addition, mitochondria show decreased mass, increased colocalization with GFAP and altered morphology. Notably, a GFP-GFAP R239H mutant recapitulates R239C-elicited alterations whereas an R239G mutant induces a milder phenotype. Together, our results outline a deleterious cycle involving altered GFAP R239C organization, mitochondrial dysfunction, oxidative stress, and further GFAP R239C protein damage and network disruption, which could contribute to astrocyte derangement in Alexander disease.
中文翻译:

亚历山大病 GFAP R239C 突变体对脂肪氧化的敏感性增加,并引起线粒体功能障碍和氧化应激
亚历山大病是一种致命的神经系统疾病,由中间丝蛋白胶质纤维酸性蛋白 (GFAP) 突变引起,该蛋白是星形胶质细胞稳态的关键。这些突变会导致 GFAP 聚集、星形胶质细胞功能障碍和神经变性。值得注意的是,大多数已知的 GFAP 突变意味着更多亲核氨基酸(主要是半胱氨酸或组氨酸)的变化,这些氨基酸更容易氧化和脂氧化。因此,我们假设亚历山大病 GFAP 突变体对氧化或亲电损伤(在神经变性过程中经常发生)的敏感性更高,可能有助于疾病的发病机制。为了解决这一点,我们在星形胶质细胞中表达了 GFP-GFAP 野生型或有害的亚历山大病 GFP-GFAP R239C 突变体。有趣的是,在对照条件下,GFAP R239C 似乎比野生型氧化得更多,这通过其较低的半胱氨酸残基可及性和二硫键低聚物的存在增加表明。此外,在用亲电介质15-脱氧-Δ 12,14 -前列腺素J 2 (15d-PGJ 2 )处理后,GFP-GFAP R239C比GFAP野生型经历更高程度的脂氧化。重要的是,GFAP R239C 细丝组织在未经处理的细胞中发生改变,并且在暴露于氧化剂(二酰胺,H 2 O 2)或亲电子试剂(4-羟基壬烯醛,15d-PGJ 2)时比 GFAP 野生型更早且更严重地受到破坏,这会加剧 GFAP R239C聚合。此外,H 2 O 2在GFAP野生型中引起可逆改变,但在GFAP R239C表达细胞中引起不可逆损伤。最后,我们发现 GFAP R239C 表达会诱导更氧化的细胞状态,游离硫醇含量降低,线粒体超氧化物生成增加。此外,线粒体质量下降、与 GFAP 的共定位增加以及形态改变。值得注意的是,GFP-GFAP R239H 突变体重现了 R239C 引起的改变,而 R239G 突变体则诱导较温和的表型。总之,我们的结果概述了一个有害的循环,涉及 GFAP R239C 组织改变、线粒体功能障碍、氧化应激以及进一步的 GFAP R239C 蛋白损伤和网络破坏,这可能导致亚历山大病中的星形胶质细胞紊乱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号