Cell ( IF 45.5 ) Pub Date : 2022-07-30 , DOI: 10.1016/j.cell.2022.06.049 Saifeng Cheng 1 , Markus Mittnenzweig 2 , Yoav Mayshar 1 , Aviezer Lifshitz 2 , Marko Dunjić 1 , Yoach Rais 1 , Raz Ben-Yair 1 , Stephanie Gehrs 3 , Elad Chomsky 2 , Zohar Mukamel 2 , Hernan Rubinstein 1 , Katharina Schlereth 3 , Netta Reines 1 , Ayelet-Hashahar Orenbuch 1 , Amos Tanay 2 , Yonatan Stelzer 1
|
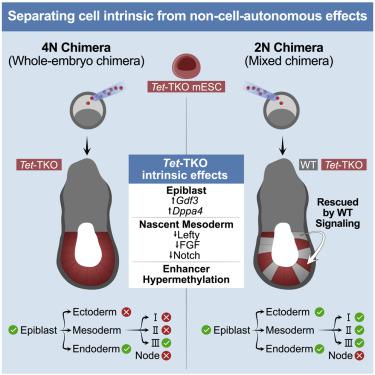
Mice deficient for all ten-eleven translocation (TET) genes exhibit early gastrulation lethality. However, separating cause and effect in such embryonic failure is challenging. To isolate cell-autonomous effects of TET loss, we used temporal single-cell atlases from embryos with partial or complete mutant contributions. Strikingly, when developing within a wild-type embryo, Tet-mutant cells retain near-complete differentiation potential, whereas embryos solely comprising mutant cells are defective in epiblast to ectoderm transition with degenerated mesoderm potential. We map de-repressions of early epiblast factors (e.g., Dppa4 and Gdf3) and failure to activate multiple signaling from nascent mesoderm (Lefty, FGF, and Notch) as likely cell-intrinsic drivers of TET loss phenotypes. We further suggest loss of enhancer demethylation as the underlying mechanism. Collectively, our work demonstrates an unbiased approach for defining intrinsic and extrinsic embryonic gene function based on temporal differentiation atlases and disentangles the intracellular effects of the demethylation machinery from its broader tissue-level ramifications.
中文翻译:

TET 蛋白在原肠胚形成过程中的内在和外在作用
缺乏所有 10-11 易位 (TET) 基因的小鼠表现出早期原肠胚形成致死性。然而,分离这种胚胎失败的因果关系具有挑战性。为了分离 TET 丢失的细胞自主效应,我们使用了来自具有部分或完全突变贡献的胚胎的时间单细胞图谱。引人注目的是,当在野生型胚胎内发育时,Tet突变细胞保持接近完全的分化潜能,而仅包含突变细胞的胚胎在外胚层到外胚层的转变中存在缺陷,具有退化的中胚层潜能。我们绘制了早期外胚层因子(例如Dppa4和Gdf3 )的去抑制图) 以及未能激活来自新生中胚层(Lefty、FGF 和 Notch)的多重信号,这可能是 TET 丢失表型的细胞内在驱动因素。我们进一步建议将增强子去甲基化的损失作为潜在机制。总的来说,我们的工作展示了一种基于时间分化图谱定义内在和外在胚胎基因功能的无偏见方法,并将去甲基化机制的细胞内效应与其更广泛的组织水平分支分开。











































 京公网安备 11010802027423号
京公网安备 11010802027423号