当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insights into Nitrate Reduction to Ammonia over Pt/TiO2: Reaction Mechanism, Activity Regulation, and Catalyst Design
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-07-30 , DOI: 10.1021/acscatal.2c01694 Zheng-Li Xie 1 , Dong Wang 1 , Xue-Qing Gong 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-07-30 , DOI: 10.1021/acscatal.2c01694 Zheng-Li Xie 1 , Dong Wang 1 , Xue-Qing Gong 1
Affiliation
|
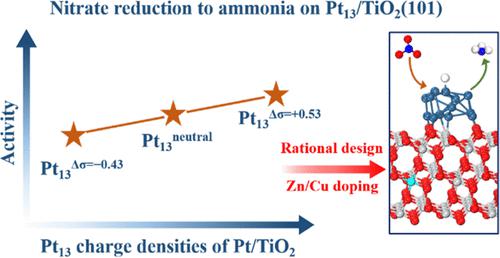
Rational design of improved catalysts is one of the ultimate goals in catalytic research, the basis of which is clarifying the reaction mechanism and regulation trends. Here, we took nitrate reduction to ammonia as an example and revealed the complete reaction mechanism, rate-determining steps, and charge density regulation trends over Pt/TiO2. The dissociation of the three N–O bonds in NO3– favors the H*-assisted pathway via HONO2*, ONOH*, and HNOH* intermediates, producing the preliminary ammonia source in the form of NH*. Subsequent hydrogenation steps of NH* + H* → NH2* + * and NH2* + H* → NH3* + * show the two largest reaction barriers, being the rate-determining steps of the reaction. Further, by regulating the Pt charge density, we showed that all of the dissociation steps are slightly deactivated, whereas the hydrogenation steps, particularly those involving NH* and NH2*, are apparently promoted as positive charges accumulate on Pt particles. Accordingly, doping of Zn or Cu into TiO2 was proposed and furthermore verified as an effective strategy to improve the nitrate reduction activity. Such a promotional effect was attributed to the reduced H* adsorption energy on the metal surface as it became positively charged, manifesting itself as a general principle in boosting the hydrogenation activity.
中文翻译:

Pt/TiO2 上硝酸盐还原为氨的理论见解:反应机理、活性调节和催化剂设计
改进催化剂的合理设计是催化研究的最终目标之一,其基础是阐明反应机理和调控趋势。在这里,我们以硝酸盐还原成氨为例,揭示了 Pt/TiO 2的完整反应机理、速率决定步骤和电荷密度调节趋势。NO 3中三个 N - O 键的解离有利于通过 HONO 2 *、ONOH* 和 HNOH* 中间体的 H* 辅助途径,产生 NH* 形式的初步氨源。NH* + H* → NH 2 * + * 和 NH 2 * + H* → NH 3的后续氢化步骤* + * 显示两个最大的反应障碍,即反应的速率决定步骤。此外,通过调节 Pt 电荷密度,我们发现所有的解离步骤都略微失活,而氢化步骤,特别是涉及 NH* 和 NH 2 * 的那些,随着正电荷在 Pt 颗粒上的积累而明显得到促进。因此,提出了将 Zn 或 Cu 掺杂到 TiO 2中,并进一步验证了它是提高硝酸盐还原活性的有效策略。这种促进作用归因于金属表面带正电时 H* 吸附能降低,这表明其本身是提高氢化活性的一般原理。
更新日期:2022-07-30
中文翻译:

Pt/TiO2 上硝酸盐还原为氨的理论见解:反应机理、活性调节和催化剂设计
改进催化剂的合理设计是催化研究的最终目标之一,其基础是阐明反应机理和调控趋势。在这里,我们以硝酸盐还原成氨为例,揭示了 Pt/TiO 2的完整反应机理、速率决定步骤和电荷密度调节趋势。NO 3中三个 N - O 键的解离有利于通过 HONO 2 *、ONOH* 和 HNOH* 中间体的 H* 辅助途径,产生 NH* 形式的初步氨源。NH* + H* → NH 2 * + * 和 NH 2 * + H* → NH 3的后续氢化步骤* + * 显示两个最大的反应障碍,即反应的速率决定步骤。此外,通过调节 Pt 电荷密度,我们发现所有的解离步骤都略微失活,而氢化步骤,特别是涉及 NH* 和 NH 2 * 的那些,随着正电荷在 Pt 颗粒上的积累而明显得到促进。因此,提出了将 Zn 或 Cu 掺杂到 TiO 2中,并进一步验证了它是提高硝酸盐还原活性的有效策略。这种促进作用归因于金属表面带正电时 H* 吸附能降低,这表明其本身是提高氢化活性的一般原理。









































 京公网安备 11010802027423号
京公网安备 11010802027423号